Abstract
The article touches upon the topical issues of a modern school the characteristic features of which are innovation and vagueness. Among the essential problem under consideration special attention is paid to the renewal of the contents and methods of teaching in modern information environment. Nowadays, being so widespread, fundamental, interdisciplinary, practice-oriented, this problem becomes one of the key issues of Russian educational system. The article shows that modern methodology for teaching subjects is focused on interdisciplinary integration of natural sciences and the humanities; these new methods of teaching are designed to guarantee the correlation between natural sciences and the humanities due to the implementation of universal modes of operation which will lead to the achievement of better results of creative and intellectual activity. The research was conducted in Secondary School 5 (in Kaluga). The teaching process was carried out in accordance with the principles of “the natural way of learning”, when the subject of study is considered from the point of view of interdisciplinary integration of the humanities and natural science that is based on the principle of minimizing the contents. The most typical objects are considered from different points of view but are analysed comprehensively. Such teaching methods contribute to deeper understanding and a better implementation of this knowledge during the creative activity.
Keywords: Subject trainingthe content of educationteaching methodsinterdisciplinary communicationthe principle of minimizationthe quality of education
Introduction
The modern world has changed. While working on new contents and methods of teaching, we cannot help taking into consideration the advantages and disadvantages of these changes closely connected with the new economic order which is influenced by the following factors:
digital cycle reduction;
the blurring of the lines between subjects and between branches of subjects;
rapid increase of scientific knowledge;
the increasing demands to the professional qualification of school leavers;
the increasing role of standards.
The exponential growth of the volume of the information that must be learnt and comprehended by students, the introduction of Federal State Educational Standards, that require the development of universal learning actions among students (for example, to determine the objectives and tasks of the education, to plan the ways of achieving these objectives during the educational process, to reflect on the process and results of education), changing requirements for the quality of the preparation of school leavers have inspired the search for more effective ways of the renovation of the educational system in the direction of blurring the boundaries (Perminova, 2015) between the natural science and the humanities, minimizing the educational contents (Gerus, 2003; Volkova, 2017), the implementation of methods which allow to master the skills of digital economics (Volkova, 2018b; Volkova & Tarakanova, 2017b).
In modern information environment in schools we observe the decline of the interest in studying natural sciences, the obsession of students with virtual communication and their unwillingness to read books. At the International conference “The results of the international research TIMSS and PISA 2015 and the factors influencing the educational system” (1st February, 2017, Moscow) the speakers stated that Russian school children do not understand scientific knowledge well enough and do not know how to apply it.
Almost all the pupils are able to observe, explain, generalize, detect, predict, draw conclusions during the experiment. A school leaver must be able to present the solutions for global problems of the world: ecological, energy, resources, they also must see the role of chemistry in solving these issues. Teachers have certain difficulties while implementing the requirements of the Federal Standard. One of the reasons is the non-systematic character of teaching methods of subjects, the lack of the correlation between natural science and the humanities while studying the same objects (Volkova, 2018a; Volkova & Tarakanova, 2017a; Volkova, Vasilieva, Tugulchieva & Khondaeva, 2018).
Problem statement
The relevance of the study of the problem of improving the quality of General education
The statement and analysis of the problem of the improvement of the quality of the education through the formation of interdisciplinary skills that are based on interdisciplinary integration concern not only a separate subject didactics, this issue is of the state importance. In the Message of President V.V. Putin to the Federal Assembly on the 1st of December, 2016 it was said: “At schools we should develop the creative ability, school children must learn to think themselves, work on their own and in a team, they also must be able to solve non-typical tasks, set goals and achieve them so that in the future it will become the basis of their wealthy and exciting life”. Modern school children pay great attention to their personal educational results: the ability to learn, to reach their objectives on their own, to acquire essential skills, “the skills of the XXI century”, their self-realization and self-development (Volkova, 2018a).
Vasilyeva, the Minister of Education, answering the questions of the TV-host of a famous TV-show “The smart ones” (“Umniki & Umnitsi”), said that “the humanities are oddly connected with natural science. No designer, technologist, physicist cannot be successful without the knowledge of the humanities. You can’t “raise” a good doctor, engineer or worker without the humanities. We know plenty of scientists who invented different things being inspired by music or art”.
The Federal State Educational Standard is focused on the following: people should live among other people and remain human. The future society will require specialist who are flexible and open-minded, have the necessary knowledge, creative enthusiasm, such character traits as the ability to adjust, decency, responsibility, self-realization, the freedom of choice, creativity, critical thinking, self-development. Education must provide each school leaver with the opportunity to gain experience in creative activity which will contribute to their self-development and will allow them to feel comfortable in the era of scientific and technological progress.
Requirements of the educational standard to graduate school
The Federal State Educational Standard of Secondary Education is designed to prepare students:
who will be able to exercise creative and critical thinking, who will enthusiastically study the world around them, who will understand the importance of science, work and creativity for a man and the society, who will be oriented towards lifelong education and self-development;
who will be able to understand the basic scientific methods of studying the world around us, who is oriented to modern creative innovative activity;
who will be ready for cooperation while studying, who will be capable of educational, research, project and information activity.
Thus, the problem of improving the quality of general education is very actual and requires urgent solution through the widespread use of interdisciplinary connections of natural-mathematical and humanitarian subjects in the process of subject education.
Research questions
Improving the quality of subject education in the modern information environment
One of the current trends in the field of research and development of Russian education is still the problem of its quality. It is due to the constantly transforming education system under the circumstances of its digitalization (Nazarova, 2012; Volkova & Tarakanova, 2016) and ever-increasing openness. There is a contradiction between the "avalanche" increase and the rapid "aging" of information, on the one hand, and the limited human capacity to consume it, on the other hand, as well as the phenomenon of globalization. Therefore, over the past 15 to 20 years, all the countries of the world are have been actively looking for new ways and means of improving the fundamentality and quality of education, constantly modernizing and transforming all the system of education (Volkova & Tarakanova, 2017a; Volkova & Tarakanova, 2017b; Volkova et al., 2018).
The appearance of new components of the content of teaching the natural science subjects related to the issues of an inter-scientific nature, requires the development of a new teaching style based on a comprehensive analysis of the substantive aspect of the educational process, based on the principle of minimization and the optimal combination of educational technologies, choice of methods, techniques, tools and organizational forms (Volkova, 2016b; Volkova & Parshutina, 2018). Interdisciplinary lessons of natural Sciences and Humanities can be one of these forms. School practice shows that subject teachers often have problems in implementing the requirements of the standard, “radical changes in teaching methods are necessary - the change from methods that focus only on mastering subject knowledge, to creating methodological systems that ensure mastering universal ways of work, achieving creative and personal education results ” (Ivanova & Serikov, 2017). As a response to the challenges of modern society, new types of activities such as teaching and research have appeared, and new form of lessons - research-lessons - have acquired a special role. At the same time, experimental and intellectual skills are formed: to plan, to model an experiment, to predict and to interpret its results, to make a hypothesis, to test it, etc. (Volkova, 2016a; Volkova, 2018b).
Approbation of research results in the practice of subject training
As an example, there is an interdisciplinary scenario on the topic “Sulfuric acid production”. The lesson is taught by a chemistry teacher together with teachers of mathematics, physics, biology, computer science and history, as the object of study is sulfuric acid, which is discussed in chemistry class, 9th form.
Plan to study the production of sulfuric acid: 1. The history of the development of the sulfuric acid production. 2. Coice of raw materials. 3. Chemistry of the process. 4. Design and operation of the main devices. 5. Choice of optimal conditions for the reactions. 6. Health and environmental protection.
After the announcement of the lesson topic the pupils are suggestered to analyse the statement of D.I. Mendeleyev: „ There is hardly other, artificially produced substance that is so commonly used in the machinery, such as sulphuric acid...“ There can be made a conclusion, that the industrial production of the sulphuric acid was very little considered at the lessons. Then 15 minutes group work at the following branches: mathematics, physics, chemistry, biology, Informatics and history, to consider one object from different points of view. The group of "historians" considers the origin of the name "vitriol oil", the history of the development of sulfuric acid production in the world and in Russia. "Mathematicians" make calculations of how much concentrated and diluted acid can be obtained from a sample of pyrite, given in the school. "Physicists" study the processes on which the production of sulfuric acid is based. "Chemists" study the reactions in the basis of sulfuric acid production by contact method. "Informatics" write a program of an expert system for the recognition of chemicals on the experimental basis, make diagrams on the production of acid in different countries and its application in different industries, analyze statistics. "Biologists" study the literature on the influence of substances that participate in the cycle of sulfuric acid production on human health.
Methodical materials on the history of sulfuric acid production
Here is the content of the first paragraph of the plan «History of development of sulfuric acid production».
Sulfuric acid has been known to mankind for a long time. It has been received for more than 1000 years. The first mention of sulfuric acid belongs to the Arab alchemist Jabir Ibn Haiyan, VIII-IX centuries. The method of obtaining sulfuric acid is described in his book "Summary of the magistery perfection”. It was received for more than 300 years, but in small amounts in glass retorts. And only in the middle of the XVIII century, as the lead was found to be highly resistant against sulfuric acid, large lead boxes or chambers were used.
The first sulfuric acid plant based on the chamber process was built in England in 1740-1746. The number of plants in England in the XVIII century began to increase due to the increase in demand for sulfuric acid from various consumers, among which a large place was occupied by textile industry. In 1750, Dr. Holm in Edinburgh found that sulfuric acid can be successfully used instead of sour milk to acidify bleached linen and cotton fabrics; as a result, the duration of this operation was reduced from 2-3 weeks to 12 hours. With the development of sulfuric acid production, work on improving the chamber process technology took place. In 1774, the French technologist De La Folie proposed to insert into the chamber not water, but water vapor, to intensify the reaction, which made it possible to conduct the process continuously. Under the influence of price increase for sulfur in 1833, the French company "Perret and son" (Lee) put to its production the pyrite firing, thereby abandoning sulfur. In 1837 the use of pyrites in the production of sulfuric acid began in Austria. In 1840, the so-called metallurgical sulfuric acid appeared in Germany, which was obtained by firing copper pyrites, lead glitter and zinc blende. This is a good example of the beginning of the combined development of metallurgical and chemical industries, which became widespread later. The next step in the improvement of sulfuric acid production was the creation of the so-called hot tower by the English technologist John. Glover in 1859. Along with the chamber method of manufacturing sulphuric acid the contact process began to develop at the end of the XIX century. It was discovered by P. Phillips (England) in 1831, who offered to oxidize sulfurous anhydride directly with air oxygen when passing the gas mixture through a heated platinum catalyst. Until the 70-ies of the XIX - early XX century contact method did not receive practical development. But the situation changed when the industry of synthetic dyes and other chemical industries developed in the 70s of the XIX century.
Job for a group of "mathematicians" and "informatics"
“Choice of raw materials” – task for the group of "mathematicians»: calculate the weight of 90% sulfuric acid, which can be obtained from pyrite, located in the school laboratory. What volume of 10% sulfuric acid solution can be prepared for practical work using the initial solution?
Additional information:
Reaction equations that can be used for calculations
4FeS2 +11O2 = 2Fe2O3 + 8SO2
2SO2 + O2 = 2SO3
SO3 + H2O = H2SO4
Some constants
M (FeS2) = 120 g/mol, М (H2SO4) = 98 g/mol
Formulas for calculations
ν (agent) = ; ω(agent) =
Using water, measure the corresponding volumes of 90% and 10% acids with a measuring Cup. For illustration tint the solution with ink.
Additional information:
ρ (90% acid) = 1,89 g/mol; ρ (10% acid) = 1 g/mol
An example of the material for the organization of the group "Informatics", which was made on the basis of educational tasks that students studied in the course of algebra, computer science and chemistry, 9th form.
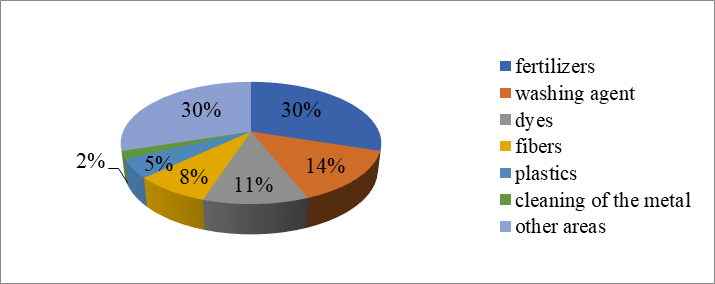
Draw a diagram in which the share of sulfuric acid from the world production for each country given in the article is shown.
Currently about 170-173 million tons of sulfuric acid is produced in the world. Over the last five years, the increase in production was about 9%. The leading place in the production of sulfuric acid belongs to the United States and China, they account for 18% of the world production as a whole. This is followed by Morocco and Russia with approximately the similar volume of output – 8.4-8.7 million tons and a corresponding share of 5%. The largest producers of sulfuric acid are also India, Japan and Brazil. From the listed main producers of sulfuric acid in the world for the period from 1999 to 2002 in countries such as China and India, acid output increased by 41% and 24%, respectively. Positive dynamics of production took place also in Russia and Brazil. In Russia, sulfuric acid production has increased by 22% over the last five years. (According to the magazine “World of sulphur, N, P and K”, 2004).
Using the diagram, determine which industry uses sulfuric acid the most. What proportion of sulfuric acid goes to the production of plastics?
Make a program to recognize sulfur dioxide and hydrogen sulfide.
Sulfur dioxide when dissolved in water forms an unstable sulfurous acid. The reaction is reversible and cannot be used for quantitative determination. However, if sulfurous anhydride is oxidized with hydrogen peroxide, a stable and strong sulfuric acid is formed. Then to the received solution to a solution of a barium salt was added. The precipitation of white milk sediment indicates the presence of sulfate ions in the solution. Hydrogen sulfide does not interfere with the definition.
To determine hydrogen sulfide, the gas is dissolved in water and a solution of lead acetate is added. In the presence of hydrogen sulfide solution precipitate from brown to black.
Tasks for the group of "physicists" and "chemists"
The group of "physicists" and "chemists" get ready to discuss the "Structure and operation of the main apparatus. The chemistry of the process", using a common text: the FIRST STAGE is the firing of pyrite in a kiln in a "fluidized bed". To obtain sulfuric acid, flotation pyrites are mainly used - production waste in the enrichment of copper ores containing mixtures of sulfur compounds of copper and iron. The process of enrichment of these ores takes place at the Norilsk and Talnakh concentrating factories, which are the main suppliers of raw materials. This raw material is more profitable, because sulfuric pyrite is mined mainly in the Urals, and, of course, its delivery can be very expensive. Perhaps the use of sulfur, which is also formed in the enrichment of ores of non-ferrous metals mined in the mines. The first stage reaction equation: 4FeS2 + 11O2 = 2Fe2O3 + 8SO2 + Q. Crushed wet cleaned (after flotation) pyrite is poured into the oven for firing in fluidized bed. From below (the counterflow principle) the air enriched with oxygen for more complete burning of pyrite is blown. The temperature in the kiln reaches 800°C. Pyrite is heated to red and is in a "suspended state" because of the air blown from below. It looks like boiling hot red liquid. Even the smallest particles of pyrite are not caked in the "boiling layer". That’s why the firing process is very fast. If earlier pyrite firing required 5-6 hours, now it takes only a few seconds. Besides, in the” fluidized bed" a temperature of 800°C can be kept. The temperature in the oven is maintained due to the released heat as a result of the reaction. Excess heat is removed: along the perimeter of the oven there are pipes with water, which is heated. The hot water is used further for central heating of the premises standing next. The resulting iron oxide Fe2O3 is not used in the production of sulfuric acid. But it is collected and sent to the metallurgical plant, where iron oxide is obtained from iron metal and its alloys with carbon - steel (2% carbon C in the alloy) and iron (4% carbon C in the alloy). Thus, the principle of chemical production-non-waste production is fulfilled. The furnace gas comes out of the oven, the composition of which is: SO2, O2, water vapor (pyrite was wet!) and tiny particles of calcine (iron oxide). Such furnace gas must be cleaned from impurities of solid particles and water vapor. Cleaning the furnace gas from the solid particles of the flame is carried out in two stages - in the cyclone (centrifugal force is used, the solid particles of the flame hit the walls of the cyclone and fall down). To remove small particles, the mixture is sent to the electrostatic precipitators, where purification occurs under the action of a high voltage current of ~ 60,000 V (electrostatic attraction is used, the scale particles adhere to the electrified plates of the electric filter, with sufficient accumulation under their own weight, they fall down), to remove water vapor in the furnace gas (drying of the furnace gas), sulfuric concentrated acidis used, which is a very good desiccant, because it absorbs water. Drying of furnace gas is carried out in the drying tower - furnace gas rises from the bottom up, and concentrated sulfuric acid flows from the top down. To increase the contact surface of the gas and liquid the tower is filled with ceramic rings. At the exit of the drying tower furnace gas no longer contains any particles of fire or water vapor. Furnace gas is now a mixture of sulfur oxide SO2 and oxygen O2.
The second stage is catalytic oxidation of SO2 in SO3 with oxygen in the contact apparatus. The reaction equation of this stage: 2SO2 + O2 ↔ 2SO3 + Q. The complexity of the second stage is that the process of oxidation of one oxide to another is reversible. Therefore, it is necessary to choose the optimal conditions for the direct reaction (to get SO3). It follows from the equation that the reaction is reversible, and, therefore, at this stage it is necessary to maintain such conditions that the equilibrium is shifted towards the output of SO3, otherwise the whole process will be disrupted. As the reaction is with a decrease in volume, it is necessary to increase the pressure - up to 7-12 psi. The reaction is exothermic, therefore, taking into account the principle of Le Chatelier, at high temperature, this process can not be conducted, as the equilibrium will move to the left. The reaction begins at a temperature of 420 degrees, but thanks to the multi-layer catalyst (5 layers), we can increase it to 550 degrees, which significantly speeds up the process. The vanadium catalyst (V2O5) is used. It's cheap, servs a long time (5-6 years), because resistant to the action of toxic contaminants. In addition, it helps shift the balance to the right. The mixture (SO2 and O2) is heated in a heat exchanger and moves through pipes, between which a cold mixture passes in the opposite direction, which must be heated. As a result, heat exchange occurs: the initial substances are heated, and the reaction products are cooled to the desired temperatures.
The third stage is the absorption of SO3 sulphuric acid in absorption tower. If water is used to absorb sulfur oxide, sulfuric acid is formed in the form of a fog consisting of tiny droplets of sulfuric acid (sulfur oxide dissolves in water with the release of a large amount of heat, sulfuric acid is so heated that it boils and turns into steam). In order to avoid the formation of sulfuric acid fog, 98% concentrated sulfuric acid is used. Two percent of the water is so little that the liquid heating will be weak and harmless. Sulfur oxide is very soluble in such acid, forming oleum: H2SO4·nSO3. Reaction equation of the process: nSO3 + H2SO4 → H2SO4·nSO3. The resulting oleum is poured into metal tanks and sent to the warehouse. Then oleum is sent to the consumer.
Job for a group of "biologists"
Materials to be analyzed by a group of "biologists" to disclose the issue of "Health and environmental protection": the Effect of hydrogen sulfide on the human body can be both positive and negative. Let’s consider how and why it is dangerous. Research on this gas began in 1998. Experiments were carried out on rats. But till now, the mechanisms of hydrogen sulfide influence on the human body and animals have not been reliably studied. Hydrogen sulfide is known to be very poisonous. Even 0.1% of the gas in the air leads to poisoning. This small concentration is able to kill within ten minutes, but the poisoning is often only expressed with symptoms. If the consentration level is higher, just one breath can be fatal. Symptoms are: headache; dizziness; nausea; high pressure. The gas has a suffocating and irritating effect on the body. It causes irritation of the eyes and respiratory tract. It is the impact on the respiratory tract which is one of the most dangerous reactions that can lead to pulmonary edema. Penetrating the body, it blocks the respiratory enzyme. Hydrogen sulphide has a detrimental effect on the protein hemoglobin. It turns the iron, which is part of hemoglobin, into iron sulfide. As a result, the blood becomes black and loses, partially or completely, the ability to carry oxygen. The gas also has a negative effect on the nervous system. Hydrogen sulfide stimulates the activity of neurons. It causes depression and anxiety. With prolonged inhalation of gas mental disorders and lesions of the autonomic nervous system may develop. Patients also suffer from insomnia. Hydrogen sulfide is a poison that, however, is able to cure. Moreover, a small amount of it is even present in our body. Scientists proved that the gas in minimum concentrations is produced in the stomach of humans and animals. The gas affects the vessels, increasing their lumen. It helps to reduce blood pressure and improves blood circulation. It has a positive effect on the nerve cells. They are damaged by free radicals that cause cancer, but the gas neutralizes them. Thus, it protects against brain damage. It is proved that by the people suffering from brain diseases, the content of hydrogen sulfide in the body is lower than normal. In addition, it stimulates the nutrition of neurons and improves memory.
Sulfur dioxide is definitely a toxic gas. Even inhalation of low concentrations can lead to inflammation of the upper respiratory tract, provoke coughing, hoarseness or rhinitis. Further exposure leads to pain during swallowing, significant speech defects, feeling of vomiting and lack of oxygen, even acute pulmonary edema. The defeat of the pulmonary tissue, at the same time, is felt not immediately, but only a day or two after the gas has penetrated the organs. Sulfur dioxide is especially dangerous for people who already suffer from respiratory diseases. For example, a person with asthma may face an allergic reaction that can lead to death. Finally, sulfur dioxide destroys vitamin B1, and completely destroys vitamin B12 in the body. Sulfur dioxide, as a preservative E220 slows down the fermentation process (with the inevitable darkening) of fresh fruits and vegetables, has the ability to bleach and preserve the "pristine" form of products. It is used as a preservative to increase the shelf life of fruit and berry juices, wines and other beverages. Alongside this, sulfur dioxide is a strong fungicide – a compound that destroys fungus that destroys wooden constructures.
Large texts help students to distribute roles in the group, to control time, to establish communication. In addition, the ability to work with large amounts of information is a requirement of time when a person finds a question of interest at a small period of time, and a team with different competencies can analyze the information, process it and provide the customer with the necessary product. In our case, to find the answer to the question, present it visually, and then present it to the class.
Results of inter-subject lesson
Then there is a group report. Students make reports about the results of the group. The groups present their answers and the audience is invited to answer the teacher's questions after the presentation. Then the teacher proposes to simulate the scheme of the acid production cycle, to compare this scheme with the given in the literature. And then to look at the photo of the real plant and try to find those devices that were named at the lesson. Students note that some of the devices have different shapes than those shown in the diagrams, that the devices are connected by a large number of different pipes, stairs and other constructions, which were not mentioned. Students conclude that there should be one more group – "designers", who should have technical thinking. Important in this lesson is the educational aspect, as environmental, economic, environmental problems of modern society are mentioned. It is concluded that the production of any substance is a branch of knowledge and industry, which combines in itself the knowledge of many branches of science. The final word of the teacher, who once again notes that in today's world it is impossible to solve problems only within one branch of science, that they are interdisciplinary. And therefore, to be a good specialist you need to have a broad outlook.
The results of the lesson are recommended in the following forms:
Cinquain is not a simple poem, but a poem written according to the following rules:
line 1 - one noun expressing the main theme of the cinquain;
line 2 - two adjectives expressing the main idea;
line 3 - three verbs describing the actions within the theme;
line 4 - phrase carrying a certain meaning;
line 5 - conclusion in the form of a noun (Association with the first word).
"Acroword" - word-arguments (phrases or sentences) beginning with the letters of the key word, arranged vertically.
Bouts-Rimes (FR. bouts-rimés - "rhymed ends") - a literary game, which consists in writing poems, often comic, on a given rhyme, sometimes even on a given topic.
Conclusion is a form of thinking that allows from one or more judgments, called assumptions, to derive a new judgment according to the rules of logic - conclusion. The conclusions distinguish between assumptions-statements that represent the original knowledge, and the conclusion - the statement to which we come as a result of the reasoning.
«In a word…» Finish with one word: I didn't know ... - now I know... .
We received the following answers from students:
group of "mathematicians": we did not know the history of sulfuric acid production, now we know it (form "in a word");
group of "physicists": how to use sulfuric acid in the best way but gently (form "Acroword");
group of "chemists": acid, charming, concentrated, produce, reacts, is used, develops the country, rich (a form of "cinquain");
group of "biologists": sulfuric acid is used in many industries, but its production requires a lot of attention (form " conclusion");
group of "informatics": sulfuric acid, poisonous, dangerous, paints, cleans, is used, cleans metals, harm or benefit (form "cinquain");
group of "historians": we learned where sulfuric acid is used (the "one word" form).
This form allowed subject teachers to improve and summarize the theoretical knowledge and subject skills of students:
In history - the formation of industrial society. The reasons for the increase in the number of discoveries in mathematics, physics, chemistry, biology, medicine in the XVIII - XIX centuries. Social effect of scientific discoveries and achievements. The role and development of education in the capitalist society. Ability to compare: economic development of Russia and Western Europe in different periods of history. Students got to know the history of the discovery of sulfuric acid, found out its main production centers and got an idea of the practical importance in the national economy and human life. Found out why in Russia the first plant for the production of sulfuric acid was opened later than in Western Europe;
in social science - market mechanisms of economic regulation. Supply and demand. Production. Factors of production. Business. Objectives of the company, its main organizational and legal forms;
In Informatics - the group worked out the construction of an information model for the chemical process of sulfuric acid production. A table model was made in Excel, which was visualized by a pie chart. The second model was made on the programming language in PascalABC environment. The analysis of the obtained models was carried out. As a result, the students worked out the stages of making a model, consolidated the skills of working with spreadsheets and programming skills in Pascal, analyzed the result of the work;
In physics - recall such phenomena as inertia, electrification, heat transfer. To revise what centrifugal force is, how charged bodies interact, how heat exchange occurs in gases. We got acquainted with the main stages of obtaining sulfuric acid, applied the knowledge gained in physics lessons;
In biology, we examined the influence of substances on organ systems from both positive and negative sides, the impact of substances on the environment.
During the collective discussion, students improved their information and communication skills, the ability to formulate and argue their own opinion. The form of the lesson contributed to the fact that each student was included in the active cognitive activity.
The teacher of history and social science faced some problems in the organization of the educational process because the content of the training had to be systematized from 5 to 9 form in history and from 7 to 8 form in social science. It was difficult for students to follow cause-and-effect connections of introduction sulfuric acid in manufacture, relying on the scientific knowledge gained on history and social science. To form the correct ideas the coordinator for students of this age should be the teacher of the subject. Thus, when making conclusions it is possible to avoid misconceptions.
The content of inter-subject class can be used for other classes, both in the system of supplementary education and at the lellons. For example, the solution of problems using the concept of "oleum" can be included in the content, as the lessons of chemistry and mathematics. For example, the solution of tasks using the concept of "oleum" can be included both into the lessons of chemistry and mathematics (Volkova, 2018c).
Purpose of the Study
The aim of the research is to consider one of the urgent problems of modern school – improving the quality of general natural science education, focused on ensuring the assimilation of universal ways of activity, the achievement of metasubject results by the students, taking into account the requirements of the GEF to the formation of students' skills to explore the world actively. The research test took place in the framework of the experimental site of the Institute of education development strategy of RAO on the basis of secondary school №5 of the city of Kaluga. Teachers of different subjects took an active part in the pedagogical experiment: biology, chemistry, physics, mathematics, computer science, history – the authors of this study. The purpose of the experiment: test of a new method of subject training, focused on the universal ways of activity, the formation of metasubject results; study of the effectiveness of the influence of interdisciplinary connections of natural mathematics and humanities on the quality of general education.
Research Methods
In the process of research the following methods were used: analysis of the didactic-methodical literature and in school practice taking into consideration the world trends of education development; comparative analysis of pedagogical experience of use different approaches to the process of subject study; experimental test of the developed technique in the conditions of the modern information environment of the school; pedagogical experiment to study the effectiveness of the influence of interdisciplinary connections of natural-mathematical and humanitarian knowledge on the quality of general education in the process of subject education.
It is very important to include into the content of natural science education the materials of modern research of natural sciences, as well as new international issues (Nazarova, 2012; Volkova & Tarakanova, 2017b; Volkova & Parshutina, 2018).
Findings
The answer to the challenges of society and the education system was the modeling of methods and technologies integrative in their nature and having synthesizing approach to the entire educational process, the transformation of educational sphere into the natural way of human life while maintaining the continuity of the educational process, held together by a unique methodological basis (Artamonov & Lovetskiy, 2004). Such methods were tested on the basis of secondary school №5 Kaluga, which is an experimental site of the Institute of education development strategy of the Russian Academy of education. The work was done by teachers of subjects of natural sciences and humanities.
Our long-term experience of school practice shows that the organization of such events allows us to individualize the education, develop cognitive interest and creativity of students (Gerus, 2003).
Conclusion
The president of the Russian Federation Vladimir Putin during a meeting with the winners of international competitions at the Sirius educational center in Sochi suggested that in the future the most popular professions will be high-tech professions, professions in the field of high technology. Vladimir Putin listed specific areas. Among them there was the industry of large numbers and their processing, artificial intelligence, robotics, genetics and everything connected with it, biology and agriculture. He also mentioned "aircraft, submarine, surface, and all sorts of unmanned vehicles". He drew attention to the fact that the greatest result is achieved by those who work at the intersection of sciences, as the boundaries between the branches of knowledge are blurred. At the same time, according to the President: "...this does not mean that the country will not need lawyers or economists. Just everything should be in moderation."
The given research can be characterized as theoretical and experimental with the bright practical orientation. The results of the research confirm the hypothesis of the possibility to achieve the goal of general education, which is to discover the abilities and ambitions of students and to get systemic experience in the process of mastering pedagogically adapted languages of national and world culture for personal, including professional, self-determination, self-actualization, self-realization and creative development in the post-industrial era and "infonosphere" civilization (Volkova, 2016a). To achieve these goals, it is necessary to give the students fundamental knowledge of the basic laws of evolution, society and science, which are the basis for any further, continuously changing knowledge.
Implementation of the mechanism of updating the content of general education and teaching methods (Gerus, 2003; Volkova, 2016b), taking into consideration the implementation of the requirements of the GEF in the modern information environment through the widespread use of interdisciplinary connections in the process of subject learning is possible in three directions:
integration of knowledge about modern research and advances in science and technology into the content of traditional school subjects (integrated education);
solving tasks of updating the content of education taking into consideration the achievements of modern science and technology in the system of additional education (extracurricular education);
the introduction of a special subject, for example, supramolecular chemistry (special education) (Volkova, 2017).
It is clear that to achieve the goal of improving the quality of general education it is necessary to look for reserves and methodological resources in interdisciplinary content. The method of teaching chemistry, focused on ensuring the assimilation of universal ways of activity, the formation of metasubject results is experimentally tested. The educational process must be built in accordance with the "natural way of learning". It is necessary to focus on the content connected with personal observations of students, with the results of their perception, systematization and understanding, as well as modeling, allowing to build models and "see" the objects of the world through the prism of academic subjects of natural science and humanitarian.
Acknowledgments
The article was implemented within the framework of the project "Updating the content of general education and teaching methods in a modern information environment". Project cipher: 27.6122.2017 / BCh.
References
- Artamonov, A.D., Lovetskiy, G.I. (2004). Technical universities in information society. In G.I. Lovetskiy, (Ed.). Moscow: Publishing house of Bauman Moscow State Technical University.
- Gerus, S.A. (2003). The theory and practice of rationalizing the process of teaching Chemistry in secondary school: monograph. St. Petersburg: Publishing House of the Herzen State Pedagogical University of Russia.
- Ivanova, S.V., Serikov, V.V. (2017). The Strategy of the development of education as a subject of interdisciplinary research. Pedagogy, 2, 3-12.
- Nazarova, T.S. (Ed.). (2012). Instrumental didactics: potential means, environment and techniques of teaching. Federal State Scientific Institution. The Institute of contents and methods of teaching. Russian Academy of Education. Moscow; St. Petersburg: Nestor-History.
- Perminova, L.M. (2015). Modern didactics: from Khomensky to present day science: philosophical and pedagogical aspects: monograph. Moscow: MIOO.
- Volkova, S. A., Vasilieva, P. D., Tugulchieva, V. S., Khondaeva, T. V. (2018). Implementation of the System Approach in Continuing Natural Science Education. Espacios, 39(38), 12. Retrieved from: http://www.revistaespacios.com/a18v39n38/18393812.html
- Volkova, S. A. (2016a). Strategy updates the content of school education in the chemical information noosphere civilization. In S. V. Ivanova, E. V. Nikulchev (Eds.). SHS Web of Conferences, 29, 01077. Retrieved from: http://www.shsconferences.org/articles/shsconf/abs/2016/07/contents/contents.html
- Volkova, S. A. (2016b). The didactics of creating school Chemistry textbook. Russian and foreign pedagogy, 2, 33-47.
- Volkova, S. A. (2017). Subject-based learning in accordance with the Federal State Educational Standard of Secondary Education: didactics. Russian and foreign pedagogy, 2, 5(44), 143-165.
- Volkova, S. A. (2018a). Interdisciplinary correlation between Chemistry and Mathematics in subject-based learning. The compendium of XII International scientific conference “Fundamental and applied issues of acquiring new data: research, innovation and techniques”, (pp. 192-196). Astrakhan: Publishing House: Sorokin Roman Vasilyevich.
- Volkova, S. A. (2018b). Formation of subject competencies in the learning of chemistry. The European Proceedings of Social & Behavioural Sciences, 46, 800-808. https://dx.doi.org/10.15405/epsbs.2018.09.02.94
- Volkova, S. A. (2018c). Theory and practice of updating the content of education in chemistry and teaching methods, in view of modern science and technology achievements. Espacios, 39(5), 32. Retrieved from: http://www.revistaespacios.com/a18v39(05)05/in183905.html
- Volkova, S. A., & Parshutina, L. A. (2018). Supramolecular chemistry as a source of the update of the school natural science curriculum. Pedagogical journal of Bashkortostan, 2(75), 15-23.
- Volkova, S. A., & Tarakanova, N. A. (2016). The influence of information-subject environment on the development of scientific literacy in chemistry among schoolchildren. Studies of Russian Academy of Education, 4(60), 51-54. Retrieved from: http://iuorao.com/setevoe-izdanie/wpuski-izdaniva.html
- Volkova, S. A., Tarakanova, N. A. (2017a). Qualitative goal as a component of Chemistry curriculum in information environment. Studies of Russian Academy of Education, 4(64), 88-91. Retrieved from: http://iuorao.com/images/UZ4_64_2017.pdfsetevoe-izdanie/wpuski-izdaniva.html
- Volkova, S. A., Tarakanova, N. A. (2017b). Methodological Guidelines For School Chemical Education Content Update in The Information-Subject Environment. The European Proceedings of Social & Behavioural Sciences, 28, 583-589. https://dx.doi.org/10.15405/epsbs.2017.08.68
Copyright information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
About this article
Publication Date
28 February 2019
Article Doi
eBook ISBN
978-1-80296-055-6
Publisher
Future Academy
Volume
56
Print ISBN (optional)
-
Edition Number
1st Edition
Pages
1-719
Subjects
Pedagogy, education, psychology, linguistics, social sciences
Cite this article as:
Volkova, S. A., Tarakanova, N. A., Ampleyenkova, E. M., Bojkova, Y. P., Smirnova, I. V., & Khritonenkova, E. L. (2019). The Correlation Between Natural Science And The Humanities At School. In S. Ivanova, & I. Elkina (Eds.), Cognitive - Social, and Behavioural Sciences - icCSBs 2018, vol 56. European Proceedings of Social and Behavioural Sciences (pp. 245-259). Future Academy. https://doi.org/10.15405/epsbs.2019.02.02.28

