Abstract
There are some reports concerning the antidepressant effects of
Keywords: OpioidergicserotonergicHypericumperforatum LantinociceptiveRat
Introduction
Pain is an unpleasant sensory and emotional experience which is developed associated with actual or potential tissue damage (Saarto, & Wiffen, 2010). Around the world, millions of people suffer from the pain and hope to find drugs with higher efficacy and fewer side effects to overcome their pain (Millan, 2002). The pain is described as one of the vital signs that lack of attention will have enormous negative consequences. Stimulation of certain areas of the brain stem can reduce or inhibit pain sensation. These areas include the abdominal area of diencephalon, periaqueductal gray matter of the brain, and the midline nuclei of brain stem (Sepehri, 2011).
Serotonin is an important mediator of pain and its important role in the pain management has repeatedly been investigated. 5HT1 and 5HT2 and 5HT3 receptors have been identified in the spinal cord and there are different and sometimes contradictory reports about the role of these receptors in pain transmission and control pain in different tests (Abe et al. 2009). Increase in the activity of serotonergic pathway is associated with increased analgesic effect and reduction of the activity of these neurons leads to increased sensitivity to painful factors (Berne et al., 2004).
Problem Statement
One of the most important factors in pain controlling is in the opioids system and analgesia induced by opioids is due to the presynaptic effects of C fiber neurons and the second degree neurons, as well as the impact on the interneurons of the spinal cord. Analgesic effects of opioids are applied through raphe Magnus nucleus and gray matter around the Sylvian aqueduct (Wagner et al. 2013). Frequent use of opioids leads to three states of tolerance, psychological dependence and physical dependence (Udenfriend, & Meienhofer, 2014).
Since standard therapies have potential side effects and no effectiveness (the use of chemical and synthetic drugs) then the use of other complementary medicines, particularly herbal treatments for pain management is increasing (Sepehri, 2011).
Research Questions
Considering that Hypericum has analgesic and antidepressant effects of and former study in which we reported analgesic effects is in the spinal cord as the main effect of the aqueous extract then the probability of analgesic effects of serotonergic and opioidergic systems in the spinal cord can be mentioned (Biranvand, & Khaksarian, 2008).
Purpose of the Study
Therefore this study aimed to investigate the role of serotonergic and opioidergic systems in the analgesic effects of aqueous extracts of
Research Methods
Animals
70 male Sprague-Dawely rats weighing 200-230 g were used for all tests.
Extract preparation method, determination of the tolerable dose range, and cannula replacing into the spinal cord and
Drugs
Methysergide (non-selective antagonists of serotonergic systems) and naloxone hydrochloride (antagonist ofopioidergic system) were purchased from Sigma Co.
Formalin test
All tests were carried out between 9 am to 5 pm at the laboratory temperature (21-23ºC) and in a relaxed and stress-free environment. 20μl formalin2.5% was injected by an insulin syringe subcutaneously into the right metatarsus. Then the animals were placed inside transparent plexiglass box with dimensions of 30*30*30cm, below which there was a 45 degree angle mirror. According to the animal dominant movement a score was given every 15 seconds and pain of animals was divided into four grades according to the following paragraphs:
0: animal sits regardless of the injected foot or walks, or stands on both legs evenly
1: The animal's foot is in contact with the compartment, but animal throws its body weight more on the healthy leg
2: Animallifts its feet completely from the floor.
3: Animal licks, chews, or severely moves the injected foot.
Zero to five minutes as the first phase (acute pain) and from 16 to 60 minutes as the second phase (chronic pain) were analyzed.
The Tail-flick test
To perform this test, the device of Tail-flick model812 made by HSE company was used. Animal were put in the Restrainer box horizontally with the hanging tail and after animal relaxed, the light intensity of 7 was used while 10 seconds was considered as cut off time to prevent tissue damage. Tail withdrawal latency was measured for each rat after light exposure (predrug-latency) and after injection (postdrug- latency) three times at intervals of 1 minute and the average response time delay (LT) was recorded. The analgesic amount created by the maximum possible analgesia (% MPE) was calculated according to the following formula.
% MPE = [(LT-BL) / 100BL] × 100
MPE (Maximum possible effect) is the maximum possible effect, BL (Base line)
Basic latency time and LT (Latency time) and Intrathecal injection drug (IT)
Methysergidedrugs were prescribed as 30 micrograms per animal and naloxone HClas 5 micrograms per animal 15 minutes before the intraperitoneal administration of aqueous extracts of
Intrathecal injection of aqueous extracts of Hp in the formalin test
The aqueous extract at doses of 1 and 2 mg per mouse and saline by volume of 10 ml were injected within one minute using a 25-ml Hamilton syringe. 10 minutes later, 50 µl of formalin 2.5% was injected into the right metatarsus andthe animals were immediately placed in the formalin test box and their pain behaviors were evaluated for 1 hour.
Intrathecal injection and ICV injection of aqueous extracts of Hpin tail flick test
At first the latency time of test was measured before treatment and then the aqueous extract and saline in doses of 1 and 2 mg/rat with a volume of 10µL within a minute using a 25-ml Hamilton syringe was injected into the spinal and intracranial. 5 minutes later, the delay time after treatment was measured through tail flick testthe ICV injection was carried out at the same way.
Each of Methysergideand naloxone hydrochloride drugs were injected for separate groups with 10 µL/min duration using the Hamilton syringe 25 ml for intrathecal injection and 15 minutes later, aqueous extracts of
Statistical analysis
Results were expressed as Mean ± SEM. Statistical calculations were conducted based on the paired t-test, unpaired t-test, one-way ANOVA, and Tukey test. P <0.05 was considered as significant level.
Findings
3.1. Sereonegic system
3.1.1. Sereonegic system in formalin test
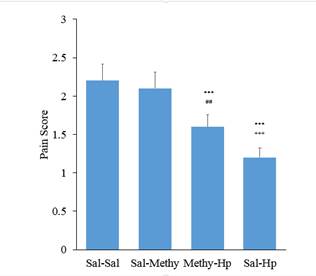
The role of the serotonergic system in the analgesic effect of
3.1.2. Sereonegic system in Tail flick test
The role of the serotonergic system on analgesic effect of
3.2. Opioidergic system
3.2.1. Sereonegic system in formalin test
The effect of Opioidergic system on the analgesic effect of Hp extract in the formalin test: intrathecal injection of naloxone 5μg/rat could substantially increase the pain in the first phase of the formalin test Figure
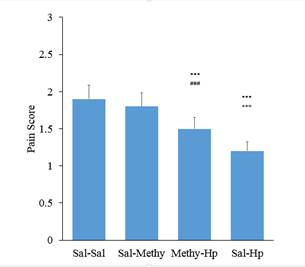
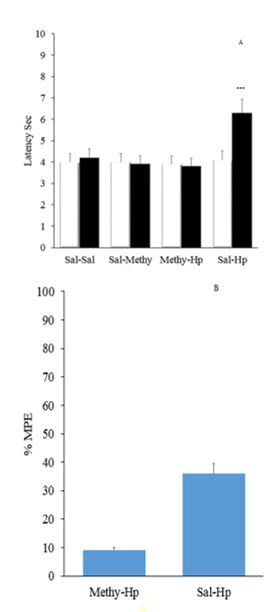
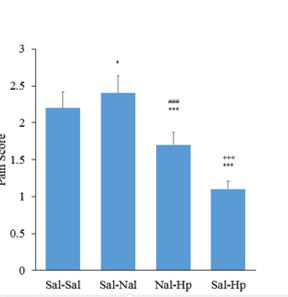
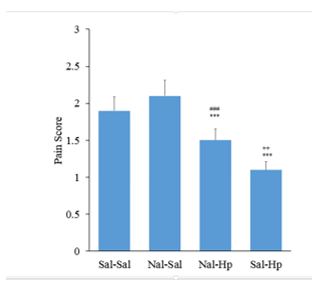
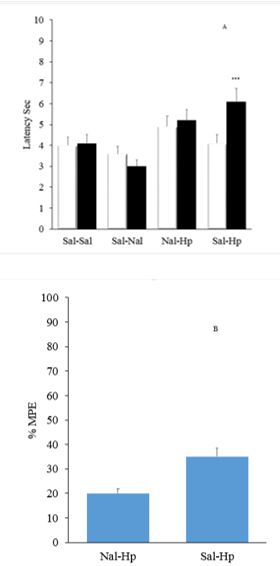
Pretreatment with intrathecal injection administration of naloxone before intraperitoneal injection of extract could not change the time delay of Tail flick test. Naloxone alone increased pain in Tail flick test. Figure
Conclusion
The results of this study showed that pain reduction at the first phase of the formalin test is mediated by serotonin receptors in some parts and by opioid receptors in other parts. Pretreatment with intrathecal injection administration of MethiSerjid before intraperitoneal injection of extract removed a part of the analgesic effect of the extract the first phase of the formalin test. This issue can suggest blocking the analgesic effects of extract through serotonergic receptor antagonists in the first phase of the formalin test. Apadins and colleagues in 1999 proposed serotonin and noradrenaline reuptake and inhibition properties for the analgesic effect of triquetrifolium Turrafrom Hypericaceae family (Sanchez-Mateo et al. 2006). 5HT1A receptor agonists reduce pain caused by the formalin (Millan, 2002). Intrathecal serotonin administration facilitates pain at lower doses and at high doses could antagonize the pain (Zeitz et al. 2002). It seems that a part of the analgesic effect of
Albert et al. (2002)showed that the extracts of
Ineffectiveness of the analgesic effect of the extract above the spinal cord obtained in the Tail flick test could be due to the different mechanisms of pain in formalin test and the mentioned test. It should be noted that the effects observed in the formalin test also confirmed more prominent spinal analgesic effects of extract in the spinal cord compared with levels above the spinal cord. MethiSerjid itself had no effect on latency of test; however pretreatment caused by intrathecal injection of MethiSerjid before injection of aqueous extracts of Hypericum intraperitoneally inhibited the analgesic effect of the extract. Therefore it can be suggested that a part of the analgesic effect of the extract in the Tail flick test is serotonergic.
On the other hand it is reported that chronic administration of
In the present study, the possible effects of other involved systems such as alpha-adrenergic system has not been investigated which is the limitation of this report; however, in another study the role of alpha-adrenergic system is studying. On the other hand, although the results of this study showed that much of the analgesic effects of
References
- Abe K, Kato G, Katafuchi T, Tamae A, Furue H, Yoshimura M. (2009). Responses to 5-HT in morphologically identified neurons in the rat substantia gelatinosa in vitro. Neuroscience. 159(1):316-24.
- A L, N G. (1995). The Complete Guide To Homyopathy. Dorling Kindersley.
- Albert D, Zündorf I, Dingermann T, Müller WE, Steinhilber D, Werz O. Hyperforin is a dual inhibitor of cyclooxygenase-1 and 5-lipoxygenase. Biochemical pharmacology. 2002;64(12):1767-75.
- Berne, R, Levy, M., Koppen, BM, Stanton, BA. (2004). Physiology, fitthed Philadelphia: mosby. 371.
- Biranvand, F, Khaksarian, M. (2008). The effects of peripheral and central administration of hypericum perforatum L and the role of alpha-adernergic system. Annals of General Psychiatry. 7(S1):S356.
- Butterweck, V. (2003). Mechanism of action of St John’s wort in depression. CNS drugs. 17(8):539-62.
- Campos AR, Santos FA, Rao VS. (2006). Ketamine-induced potentiation of morphine analgesia in rat tail-flick test: role of opioid-, α2-adrenoceptors and ATP-sensitive potassium channels. Biological and Pharmaceutical Bulletin. 29(1):86-9.
- Dimpfel, W, Hofmann, R. (1995). Pharmacodynamic effects of St. John's Wort on rat intracerebral field potentials. European journal of medical research. 1(3):157-67.
- Dimpfel, W, Todorova, A, Vonderheid-Guth, B. (1999). Pharmacodynamic properties of St. John's wort-A single blind neurophysiological study in healthy subjects comparing two commercial preparations. European journal of medical research. 1999;4(8):303-12.
- Dulu, TD., Kanui, TI., Towett, PK., Maloiy, GM., Abelson, KS. (2014). The effects of oxotremorine, epibatidine, atropine, mecamylamine and naloxone in the tail-flick, hot-plate, and formalin tests in the naked mole-rat (Heterocephalus glaber). in vivo. 28(1):39-48.
- Jean, D, Pouligon, M, Henriot, A. (2006). Pharmacological activity of three commercial Hypericum perforatum preparations in mice. Phytotherapy Research. 20(8):653-4.
- Kaehler, ST, Sinner, C, Chatterjee, SS, Philippu, A. (1999). Hyperforin enhances the extracellular concentrations of catecholamines, serotonin and glutamate in the rat locus coeruleus. Neuroscience letters. 262(3):199-202.
- Khaksarian, M, Javan, M, Sonboli, A, Motamedi, F. (2004). Inhibition of acute and chronic pain in male rats by aqueous extract of Hypericum perforatum L.
- Kümper, H. (1989). Hypericum poisoning in sheep. Tierärztliche Praxis. 17(3):257.
- Levine, J., Gordon, N., Jones, R., Fields, H. (1978). The narcotic antagonist naloxone enhances clinical pain.
- Lunzer, MM., Yekkirala, A., Hebbel, RP., Portoghese, PS. (2007). Naloxone acts as a potent analgesic in transgenic mouse models of sickle cell anemia. Proceedings of the National Academy of Sciences. 104(14):6061-5.
- Millan, MJ. (2002). Descending control of pain. Progress in neurobiology. 66(6):355-474.
- Nathan, PJ. (2001). Hypericum perforatum (St John's Wort): a non-selective reuptake inhibitor? A review of the recent advances in its pharmacology. Journal of Psychopharmacology. 15(1):47-54.
- Obach, RS. (2000). Inhibition of human cytochrome P450 enzymes by constituents of St. John's Wort, an herbal preparation used in the treatment of depression. Journal of Pharmacology and Experimental Therapeutics. 2000;294(1):88-95.
- Perfumi, M, Panocka, I, Ciccocioppo, R, Vitali, D, Froldi, R, Massi, M. (2001). Effects of a methanolic extract and a hyperforin-enriched CO2 extract of Hypericum perforatum on alcohol intake in rats. Alcohol and Alcoholism. 36(3):199-206.
- Raak, C, Büssing, A, Gassmann, G, Boehm, K, Ostermann, T. (2012). A systematic review and meta-analysis on the use of Hypericum perforatum (St. John's Wort) for pain conditions in dental practice. Homeopathy. 101(4):204-10.
- Saarto, T, Wiffen, PJ. (2010). Antidepressants for neuropathic pain: a Cochrane review. Journal of Neurology, Neurosurgery & Psychiatry. 81(12):1372-3.
- Sanchez-Mateo, C., Bonkanka, C., Hernandez-Perez, M., Rabanal, R. (2006). Evaluation of the analgesic and topical anti-inflammatory effects of Hypericum reflexum L. fil. Journal of ethnopharmacology. 107(1):1-6.
- Sepehri, G, Sheibani, V, Pahlavan, Y, Afarinesh, Khaki, M, Esmail, Pour Bezenjani, K, Pahlavan, B. (2011). Effect of Interacerebroventricular Injection of Aqueous Extract of Origanum Vulgare L. ssp. viride on Pain Threshold in Male Rats. Journal of Ardabil University of Medical Sciences. 11(1):52-8.
- Trovato A, Raneri E, Kouladis M, Tzakou O, Taviano MF, Galati EM. (2001). Anti-inflammatory and analgesic activity of Hypericum empetrifolium Willd.(Guttiferae). Il Farmaco. 56(5):455-7.
- Udenfriend, S., Meienhofer, J. (2014). Opioid Peptides: Biology, Chemistry, and Genetics: The Peptides: Analysis, Synthesis, Biology: Elsevier.
- Wagner, K, Roeder, Z, DesRochers, K, Buhler, AV, Heinricher, MM, Cleary, DR. (2013). The dorsomedial hypothalamus mediates stress-induced hyperalgesia and is the source of the pronociceptive peptide cholecystokinin in the rostral ventromedial medulla. Neuroscience. 2013;238:29-38.
- Zeitz KP, Guy N, Malmberg AB, Dirajlal S, Martin WJ, Sun L, et al. (2002). The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. Journal of Neuroscience. 22(3):1010-9.
Copyright information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
About this article
Publication Date
14 September 2017
Article Doi
eBook ISBN
978-1-80296-029-7
Publisher
Future Academy
Volume
30
Print ISBN (optional)
-
Edition Number
1st Edition
Pages
1-376
Subjects
Health, public health, preventive healthcare, preventive care, preventive medicine
Cite this article as:
Khaksarian, M., Sonboli, A., Javan, M., Mahmoudvand, H., Nazari, M., & Motamedi, F. (2017). Hypericumperforatum L. Showed Antinociceptive Effects Through Opioidergic And Serotonergic Systems. In Z. Bekirogullari, M. Y. Minas, & R. X. Thambusamy (Eds.), Health and Health Psychology - icH&Hpsy 2017, vol 30. European Proceedings of Social and Behavioural Sciences (pp. 35-46). Future Academy. https://doi.org/10.15405/epsbs.2017.09.4
