Abstract
OBJECTIVE: The aim of the study is to observe the effects of active, guided exercises on postoperative complications, shoulder range of motion (ROM) and functionality of the arm and the in women undergoing radical mastectomy or lumpectomy with axillary lymph node dissection for breast cancer. We compared patients who participated at the recovery exercise program to those who didn't attend any exercise program and patients with different associated therapies (hormone, chemo, chemo+radiotherapy). METHODS: The study was carried out on 140 patients with breast surgery for cancer, divided in two groups: the study group and the control group. Both groups were divided in three categories: patients with associated hormone therapy, patients with chemotherapy and patients with chemotherapy and radiotherapy. RESULTS: The most affected movements after surgery are: flexion and abduction. Also, the most significant changes happen for these two movements after daily participating to the physical therapy program. Postoperative complications are ameliorated and functionality tests for the arm show an improvement. CONCLUSIONS: A structured, patient tailored, well designed and early applied exercise program has several benefits on troublesome symptoms, functionality and range of motion of the ipsilateral arm. There are differences between the three categories of patients regarding the followed aspects, although, physiotherapy influences all of those aspects in well.
Keywords: Physiotherapybreast cancershoulder joint
Introduction
Breast cancer and its treatment have common side effects such as: decreased functional capacity
and range of motion (ROM), fatigue, depression, lymphedema, decreased muscle strength tightness, pain,
numbness of the arm, fibrous and lymphatic cords, rigidity, decreased tolerance to activities and
limitations in daily life (de Oliveira & al. 2014; Beurkens & al. 2007; Torres & al. 2010). These
complaints could be due to tissue and nerve damage. Altered body alignment is frequently encountered
and it is caused by redistribution of the weight on the spine, the body loses symmetry following unilateral
breast resection (Puscas & al. 2013). In general, the arm-related complaints usually decrease within three
months (Beurskens & al, 2007). But for many breast cancer survivors, upper-limb dysfunction may
persist for many years and affect quality of life. Shoulder exercises commonly are prescribed for patients
to prevent or minimize these side effects (Hu & Zhou, 2011).
The major postoperative complication of the treatment of breast cancer is the limitation of the
range of motion (ROM) of the shoulder and it is accompanied by impairment and decrease of the upper
limb function (Petito & al, 2012). Most affected movements after surgery are: flexion, abduction and
external rotation (Hsieh & al. 2008).
Immediate postoperative complications related to surgical wound are: seroma, wound dehiscence,
infection and hematoma. These complications occur in about 30 % of cases (Petito & al. 2014).
The treatment of breast cancer includes, beside of surgery, endocrine (hormone) therapy,
chemotherapy and/or radiation therapy. All of these treatments have disturbing side effects. Hormone
treatment may cause hot flashes, sleep disturbances, loss of bone mineral density (BMD), and muscle and
joint pain. In addition to physical symptoms related to treatments, psychological symptoms such as
depression and anxiety associated with the diagnosis of a serious illness may be present (Binkley & al.
2012).
Chemotherapy for breast cancer often is associated with alopecia, fatigue, neuropathy, nausea, and
muscle and joint pain. Chemotherapy often leads to early onset of menopause in younger women which,
in turn, may be associated with other long-term consequences such as BMD loss and weight gain
(Binkley & al. 2012).
Radiation therapy can produce skin irritations and a contracture of cicatrices (Listing & al. 2009).
For a complete picture of postoperative motor deficit that requires correction and disturbing
symptoms that may occur after surgery or/and anaesthesia, over which it may act by physiotherapy,
perioperative evaluation is essential.
The bio-psycho-social benefits of moderate intensity and customized (Hsieh & al. 2008) exercise
program for breast cancer patients are well known, including improvement of cardiorespiratory and
muscular fitness and quality of life (McNeely & al. 2006; Courneya, 2003; Cheema & Gaul, 2006;
Visovsky & al, 2006; Valenti & al, 2008; Garder & al, 2015). Caution is advised only when
chemotherapy causes blood count change or other physical symptoms (Adkins, 2009; Courneya & al.
2002). Exercise can boost or reduce the resolution of comorbidities associated symptoms of breast cancer
(Adkins, 2009).
Exercise therapy is beneficial during adjuvant therapy, for example, it may help to reduce the
frequently appearing fatigue symptoms (Mocket & al. 2001). Recent research indicates that exercise
training during and after treatment may prevent and reduce cancer-related fatigue complaints (Travier &
al, 2015). Resistance exercise increases bone density (Adkins, 2009). Due to this fact, it is particularly
beneficial to patients under chemotherapy-induced menopause, and with an increased risk of
osteoporosis. By improving body mass index and muscle mass, reduces the risk of falls and fracture
(Adkins, 2009).
Structured exercise programs are helpful in regaining shoulder mobility and functional capacity in
the early weeks following surgery without causing adverse side effects (Hu & Zhou, 2011).
Regular physical exercise may lead to a reduction in excessive body mass and mood improvement,
lowering anxiety and depression and increasing the threshold of emotional stress. Daily aerobic exercise
is associated with positive changes on body composition and quality of life of breast cancer patients.
After the implementation of combined training (aerobic and resistance), further improvement of physical,
emotional, social and role functioning may be observed (Hojan & al. 2013). High physical activity levels
and a healthy body weight is associated with better quality of life after breast cancer (Voskuil & al. 2010;
Schwartz & al. 2007; Abrahamson & al. 2006; Holmeset & al. 2005, Pierce & al. 2007).
To decrease the incidence of the reported complications, studies highlight that the performance of
exercises immediately after surgery gives positive results, both in the physical and the psychological
areas, since it provides conditions for the woman to return to their activities of daily living (ADLs) within
a shorter period of time. The majority rehabilitation programs recommends sessions in which women
undergoing surgery perform exercise in the physiotherapy or rehabilitation service of the hospital, often
two to three times a week, complemented at home, with the help of manuals and/or educative videos. Few
studies, however, report on exercise performed exclusively in the domicile (Petito & al. 2012; Kilgour &
al. 2008; Amaral & al. 2005).
In common, all the programs present a preoperative evaluation and a gradual progression in the
exercises, starting early, from the first postoperative day (Cinar & al. 2008, Kilgour & al. 2008; Springer
& al, 2010; Pinto & al. 2004). Only one study recommends the initiation of the exercise program after
removal of the drain (Amaral & al, 2005), and another later, after the 6th or 26th postoperative week
(Lauridsen & al. 2005).
The established program will try to fulfil the patient’s needs and will be systematically reviewed to
be adapted for the changes that occur. It is recommended that the recovery program to contain aerobic
type exercises, moderate, 3-5 days per week for 20-60 minutes (Courneya & al. 2002). Dosage of the
exercise program will be customized for each patient, considering their age, activity level before
diagnosis and medication therapy for associated diseases. The application of physiotherapy during the
first postoperative week is important also in order to show the patients that they are allowed to use the
shoulder (Lauridsen & al. 2005).
Another benefit of the exercise program is the improvement of the lymphatic system functioning
(Thakur & al, 2016). The literature suggests that active exercise stimulates musculoskeletal contractions,
which may be considered a major pumping mechanism for lymphatic and venous drainage (Bicego & al, 2006). Also, active exercise plays a key role in developing new pathways for lymphatics (Lane & al. 2005) and promoting lymphangiogenesis (de Oliveira & al. 2014).
No evidence exists of adverse effects on incidence of seroma formation, pain, lymphedema and
delayed wound healing. Early exercise may cause more wound drainage, so patients must be informed of
that drains should be kept in place longer. (Hu & Zhou, 2011).
The application of additional physiotherapy during radiotherapy or shortly after, encourage the
patients to use the shoulder in full scale. The extension of the scar tissue and the muscles reduces the firm
attachment of the skin to the underlying tissue and reduces the shortening of the muscles. Hence, the
shoulder mobility is improved (Lauridsen & al. 2005).
Massage therapy is also part of the recovery program. Massage treatment significantly reduced
some of the disturbing symptoms, especially from the breast level. The reduction in breast symptoms
might be explained by reduced myofascial trigger point sensitivity, an increase in blood and lymph flow,
or an influence on the autonomic nervous system. If in the massage therapy the pressure on the affected
area and contraindications such as acute thrombosis, inflamed skin in the area of therapy were excluded,
this treatment is considered to be safe and side effects for example hematomas are rare (Listing & al.
2009).
Problem statement
Treatment procedures for breast cancer has several side effects, which untreated, exacerbate.
Research questions
How can a structured, patient-tailored regular exercise program reduce the treatment’s side effects,
during the patient’s hospitalization?
Purpose of the study
Studying the effects of an exercise program in women who performed surgery for breast cancer
permits us to develop recovery program protocols.
Research Methods (Matherial and Methods)
This study was carried out on 140 patients with breast cancer following modified radical
mastectomy in the Surgery Unit of the Institute of Oncology from Cluj-Napoca between November 2010
and March 2012.
Selection criteria were: modified radical mastectomy, age between 40-65, normal BMI and
ultrasound to exclude recurrence.
Exclusion criteria were: cancer recurrence, untreated/unsolved infection, untreated congestive
heart failure, kidney failure, deep vein thrombosis of the arm or difficulties in understanding the
physiotherapist’s indications/ psychiatric disorders.
We grouped the patients, by their expressed option in two: an interventional group, consisting of
70 patients and the control group, also consisting of 70 patients.
The study group includes:
Lot I: 33 patients with endocrine therapy,
Lot II: 25 patients with chemotherapy before breast surgery,
Lot III: 12 patients who had undergone both chemo and radiation therapy before breast surgery.
The witness group includes:
Control I: 32 patients with endocrine therapy,
Control II: 25 patients with chemotherapy
Control III: 13 patients with chemo and radiation therapy.
Indicators:
The range of motion of the shoulder was measured before and every day after surgery with a
internal and external rotation were noted.
The patients were asked to take a global functional test, consisting in four items: hand to the
cervico-dorsal region; hand to the subscapular region; a tray in the hands and a cup to the mouth.
Besides the reassessment of the mentioned indicators, we inventoried the following symptoms:
Pain (where 1 means not at all, and 5 means unbearable pain without painkillers) – by patient’s
response,
Tingling (1= not at all, 2 presence) - by patient’s response,
Paraesthesia (1= not at all, 2= presence) - by patient’s response,
Edema (1= not at all, 2= presence) - by measuring with a metric tape,
Vasomotor disturbances (1= not at all, 2= presence) – by measuring the temperature of both hands,
with a thermometer, expressed in°Celsius.
Moments of evaluation: the study group was evaluated before surgery and every day after surgery,
before the exercise program; the control group was evaluated before surgery and the 5-th day after
surgery, at discharge.
The recovery program, consisting of exercises and massage therapy was initiated first day after
surgery, after the surgeons evaluated the patients and gave their approval for starting the recovery
sessions. All of the patients were evaluated by a physiotherapist before every session.
The day of the surgery, after 4-6 hours after orotracheal detubation, we started the physiotherapy
program with respiratory facilitating postures. To the postures we associated thoracic percussions and
vibrant massage. As physical exercises, we initialized the respiratory reeducation, for the thoraco-
abdominal respiration and for the body alignment. Relaxation and isometric contraction of the muscles
are used, especially for the upper body, followed by passive, passive-active and active movements.
Active stretching was introduced from day 3 after surgery. Active movements with light resistance
were introduced after four days from surgery.
Every day patients attend a supervised class in the morning and a self-administrated exercise
program in the evening, following the written indications received by the physiotherapist.
The exercise program was individualized to each patient. Preferences were inventoried during the
first exercise session. Also the fitness level was assessed by means of a cardiopulmonary exercise test and
1-repetition maximum muscle strength tests. (Travier & al. 2015). Muscle strength training was
performed for all major muscle groups: arms, legs, shoulder, and trunk. The training started with 1 × 6
repetitions and gradually increased to 1 × 10 repetitions and the goal was to reach 2 × 10 repetitions by
the end of the program. Also, patients perform the exercises from the
Light pressure effleurage massage of the neck, head, arms and back was gave by the
physiotherapist the second and the fourth day after surgery.
Every exercise was stopped at first sign of discomfort, pain, dizziness, weakness or nausea.
The control group was encouraged to move their arm, but no specific guidance was offered.
The patients were observed for five days after the surgery, during their hospitalization.
Ethical standards
We mention that the patients gave their informed consent in writing for the study and that we
obtained the approval of the Medical Ethics Committee of this institution.
Statistical analysis
Statistical analysis was performed using Statistica 8.0 for Windows (Stat-Soft, Inc., USA). Box-
and-whisker plots, t-test and non-parametric tests (Wilcoxon matched pairs test, sign test, 2x2 table
evaluation) were used to examine the strength of association between results. The experimental data were
evaluated using one-way analysis of variance (ANOVA), with p < 0.05 as threshold for statistical
significance. The statistical results confirm the hypothesis that the differences between the results are
either not significant (p > 0.05), significant (0.001 < p < 0.05) or highly significant (p < 0.001). The mean
value was used for the plots and the box and whiskers are the standard error of the mean and standard
deviation, respectively.
Findings (Results)
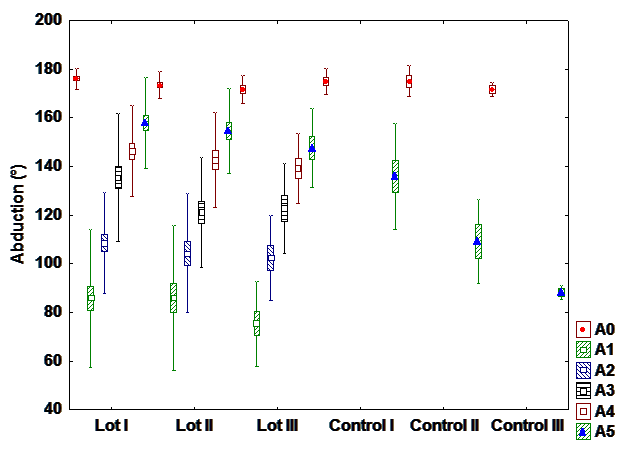
The most affected movements after surgery are: abduction and flexion. The most commonly reported
symptoms include: pain, swelling, paraesthesia and vasomotor disturbances.
a). Abduction
After statistical analysis, a statistically significant difference is observable between the patients who
had hormone therapy from the interventional and control group. This indicates a positive influence of
exercise program on the recovery of the shoulder’s range of motion.
A highly statistical significance is observed between patients with chemotherapy between the
interventional and the control group. Physical therapy determines the recovery of abduction and improves
the side effects of chemotherapy.
There is a highly statistical significance between the group with chemo and radiotherapy and
interventional control group with the same treatment.
It can’t be observed a statistically significant difference in the fifth postoperative day (at discharge)
between interventional groups; the percentage recovery of the abduction is very similar between patients
with hormone therapy, chemotherapy and radio-chemotherapy (Fig. 1).
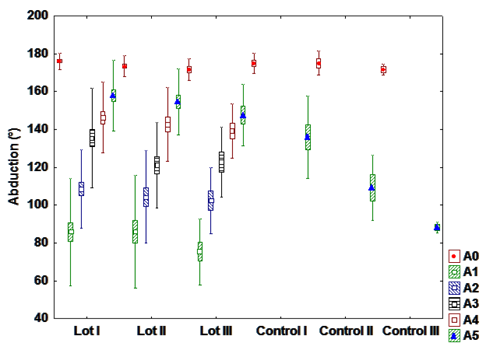
b). Flexion
There is a statistically highly significant difference between interventional and control groups in
patients with hormone therapy and chemotherapy, and a highly significant difference between patients
with radiation and chemotherapy from the interventional and control group, meaning that patients who
have undergone rehabilitation program presents a much wider range of motion relative to patients who
have not been directed to exercise.
There is a highly significant statistical difference in patients from the interventional groups, between
those who have only hormone therapy and those with radiation-chemotherapy. This is explained by
adverse effects of radiation and chemotherapy, which restrict the range of motion and affects negatively
the recovery rate (Fig. 2).
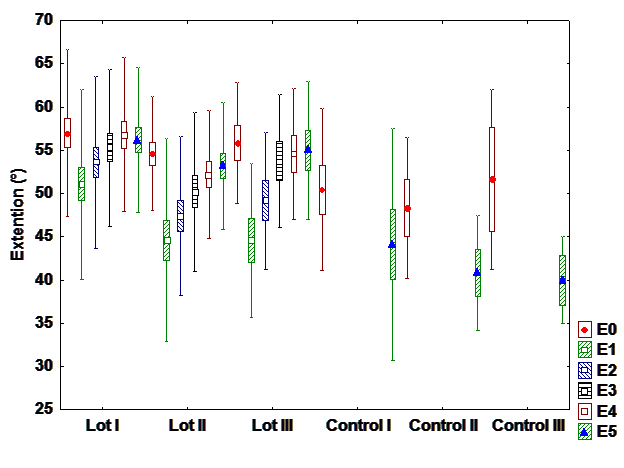
c). Extension
The extension is less affected movement after surgery. However, it can be observed a statistically
significant difference between interventional and control groups. There are also differences between
groups by treatment. Recovery is linear and positive. (Fig 3).
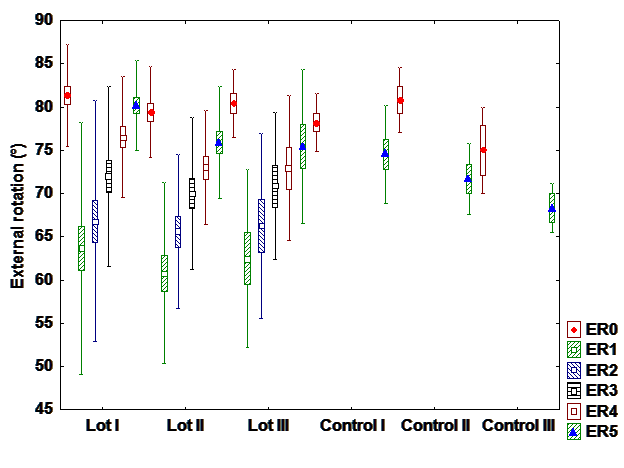
d). Iternal rotation
There is a difference between interventional and control group with hormone therapy, but there
are no differences between groups in patients with chemo and radiation+chemotherapy. It can be
observed differences between patients with different treatments. Most patients with hormone therapy
recover entirely the internal rotation while patients with radiation and chemotherapy patients recover the
least. (Fig 4).
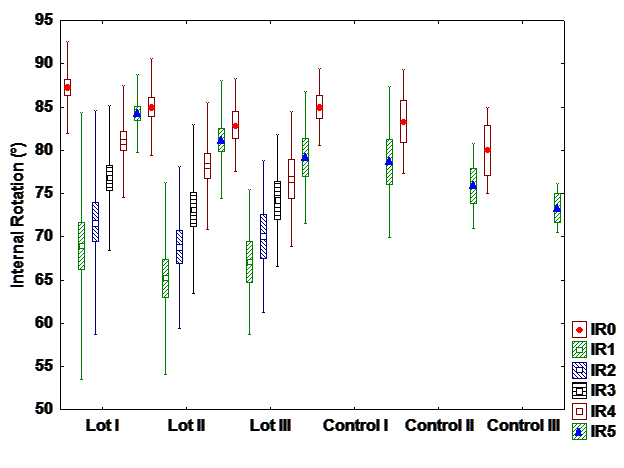
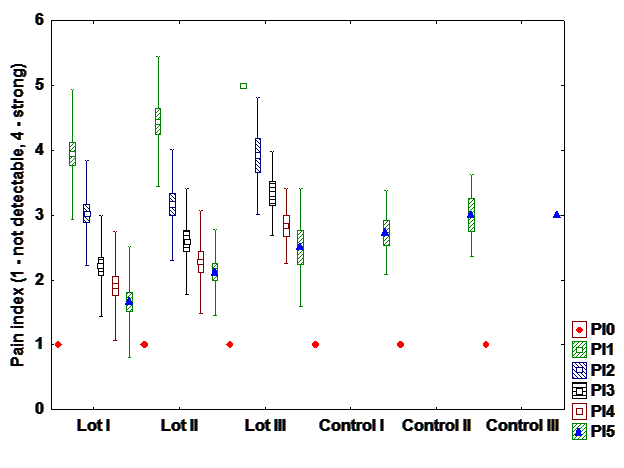
e). Pain index
Regarding pain, patients presents an exponential recovery. On the first day almost all patients are
affected, but on the fifth day the pain is much improved and is no longer reported, particularly in patients
who undergo hormone therapy only. (Fig. 6)
f). Edema
Among patients with chemotherapy, in the first postoperative day, is observed most cases of
swelling in the arm from the operated side. On the fifth day, the patients in the interventional group with
hormone therapy and chemotherapy presents a complete recovery, while in patients with radiation and
chemotherapy, edema persists. It is assumed that radiation therapy is one that predispose to lymphedema.
g). Paresthesia and vaso-motor disorders
There is a highly- highly statistical significance between the interventional and control group. Physical
therapy significantly improves symptoms. Exercise and massage have a significant beneficial effect on
these symptoms. As regards the vaso-motor disorders in the first postoperative day, the group with
hormone therapy is less affected that the group with chemotherapy and the group with radiation and
chemotherapy. The recovery is exponential.
h). "The hand on the nap"
All patients have problems in the first day after surgery at this functional test. Recovery presents
a positive trend, linear, with differences between interventional and control groups. There is no difference
between patients depending on the followed treatment.
i). Hand to subscapular region
In the first postoperative day there are major differences between patients based on the followed
treatments. Patients with radiation and chemotherapy patients are most affected, but at discharge, the
results are comparable. Statistically significant differences between the intervention and the control
groups can be observed.
Conclusions
The most affected movements after surgery are: abduction and flexion. The most commonly
reported symptoms include: pain, swelling, paraesthesia and vasomotor disturbances;
Regarding the recovery of the abduction, there is a great difference between the patients in the
interventional and the control groups and very small difference between interventional groups. On the
fifth day after surgery, there isn’t any significant difference between patients depending on the followed
treatmet.
There are differences in paraesthesia and vasomotor disturbances between interventional and the
control groups. Recovery is exponential; physical therapy has a positive influence on improving these
symptoms.
Regarding functional test "hand on the nape", all patients were experiencing problems on the first
day after surgery at this functional test. Recovery has a positive trend, linear, with differences between
interventional and control groups. There is no difference between patients depending on the followed
treatment.
Copyright
This paper is original, has been written by the stated authors and has not been published elsewhere.
The article is not currently being considered for publication by any other journal and will not be
submitted for such review while under review by Future Academy. No funding was received for the
research reported in the article. There is no conflict of interests regarding this study.
Acknowledgements
The authors would like to thank all patients for participating and collaborating with the therapeutic team.
References
- Abrahamson, P.E., Gammon, M.D., Lund, M.J., Britton, J.A., Marshall, S.W., Flagg, E.W. & Coates, R.J. (2006). Recreational physical activity and survival among young women with breast cancer. Cancer, 107, 1777–1785, doi: 10.1002/cncr.22201.
- Adkins, B.W. (2009). Maximizing exercise in breast cancer survivors. Clin J Oncol Nurs, 13(6):695-700, doi: 10.1188/09.CJON.695-700.Ruismäki, H. & Tereska, T. (2006). Early childhood musical experiences: contributing to pre-service elementary teachers’ self-concept in music and success in music education (duringstudent age). European Early Childhood Education Research Journal, 14(1), 113-130.
- Amaral, M.T.P., Teixeira, L.C., Derehain, S.F.M., Nogueira, M.D., Pinto e Silva, M.P. & Gonçalves, A.V. (2005). Orientaçâo domiciliar: proposta de reabilitaçâo física para mulheres submetidas à cirurgia por câncer de mama. Rev Cieñe Méd, 14(5):405-13.
- Beurskens, C.H., van Uden, C.J., Strobbe, L.J., Oostendorp, R.A. & Wobbes, T. (2007). The efficacy of physiotherapy upon shoulder function following axillary dissection in breast cancer, a randomized controlled study. BMC Cancer, 7: 166.
- Bicego, D., Brown, K., Ruddick, M., Storey, D., Wong, C.n & Harris, S.R. (2006). Exercise for women with or at risk for breast cancer–related lymphedema. Physical Therapy, 86:1398–1405.
- Binkley, J. M., Harris, S. R., Levangie, P. K., Pearl, M., Guglielmino, J., Kraus, V., & Rowden, D. (2012). Patient perspectives on breast cancer treatment side effects and the prospective surveillance model for physical rehabilitation for women with breast cancer. Cancer (0008543X), 1182207-2216, doi:10.1002/cncr.27469.
- Cheema, B.S. & Gaul, C.A. (2006). Full-body exercise training improves fitness and quality of life in survivors of breast cancer. J Strength Cond Res, 20:14–21.
- Cinar, N., Seckin, U., Keskin, D., Bodur, H., Bozkurt, B. & Cengiz, O. (2008). The effectiveness of early rehabilitation in patients with modified radical masteetomy. Cancer Nurs, 31(2):160-5.
- Courneya, K.S. (2003). Exercise in cancer survivors: An overview of research. Med Sci Sports Exerc2003; 35:1846–1852, doi: 10.1249/01.MSS.0000093622.41587.B6.
- de Oliveira, M. F., de Rezende, L. F., do Amaral, M. P., Pinto e Silva, M. P., Morais, S. S., & Costa Gurgel, M. S. (2014). Manual lymphatic drainage versus exercise in the early postoperative cancer. Physiotherapy Theory & Practice, 30(6), 384-389, period for breast doi:10.3109/09593985.2013.876695.
- de Rezende, L.F., Franco, R.L., Rezende, M.F., Beletti, P.O., Moráis, S.S & Gurgel MS. (2006). Two exereises schemes in postoperative breast cancer: comparison of effects on shoulder movement and lymphatic disturbance. Tumori, 92(l):55-61.
- Harder, H., , Langridge, C., Solis-Trapala I., Zammit, C., Grant M., Reesd, D., Burkinshaw, L., Jenkins, V. (2015). Post-operative exercises after breast cancer surgery: Results of a RCT evaluating standard care versus standard care plus additional yoga exercise. Eu J Integr Med, 7(3):202–210, doi:
- Hojan, K., Molińska-Glura, M., & Milecki, P. (2013). Physical activity and body composition, body physique, and quality of life in premenopausal breast cancer patients during endocrine therapy - a feasibility study. Acta Oncologica, 52(2), 319-326, doi:
- Holmes, M.D., Chen, W.Y., Feskanich, D., Kroenke, C.H. & Colditz, G.A. (2005). Physical activity and survival after breast cancer diagnosis. JAMA, 293:2479–2486. Retrieved from http://jama.ama-assn.org/cgi/content/full/293/20/2479
- Hsieh, C.C., Sprod, L.K., Hydock, D.S., Carter, S.D., Hayward, R & Schneider, C.M. (2008). Effects of a supervised exercise intervention on recovery from treatment regimens in breast cancer survivors. Oncol Nurs Forum, 35(6):909-15, doi: 10.1188/08.ONF.909-915.
- Hu, C., & Zhou, L. (2011). Exercise Interventions for Upper-Limb Dysfunction Caused by Breast Cancer Treatment. Clinical Journal Of Oncology Nursing, 15(5), 569-570.
- Kilgour, R.D., Jones, D.H. & Keyserlingk JR. (2008). Effectiveness of a self-administered, home-based exercise rehabilitation program for a women following modified radieal masteetomy and axillary node dissection: a preliminary study. Breast Cancer Res Treat, 109(2):285-95.
- Lane, K., Worsley, D., McKenzie, D. (2005). Exercice and the lymphatic system – Implications for breast-cancer survivors. Sports Medicine, 35: 461–471.
- Lauridsen, M.C., Christiansen, P. & Hessov, I. (2005). The effect of physiotherapy on shoulder function in patients surgically treated for breast cancer: A randomized study. Acta Oncologica, 44(5), 449-457, doi:10.1080/02841860510029905.
- Listing, M., Reißhauer, A., Krohn, M., Voigt, B., Tjahono, G., Becker, J. & Rauchfuß, M. (2009). Massage therapy reduces physical discomfort and improves mood disturbances in women with breast cancer. Psycho-Oncology, 18(12), 1290-1299, doi:10.1002/pon.1508.
- McNeely, M.L., Campbell, K.L., Rowe, B.H., Klassen, T.P., Mackey, J.R. & Courneya KS. (2006). Effects of exercise on breast cancer patients and survivors: a systematic review and metaanalysis. CMAJ, 175:34–41.
- Mock, V., Pickett, M. & Ropka, M.E (2001). Fatigue and quality of life outcomes of exercise during cancer treatment. Cancer Pract, 9:119–127.
- Petito, E., Nazário, A., Martinelli, S., Facina, G.& De Gutiérrez, M. (2012). Application of a domicile-based exercise program for shoulder rehabilitation after breast cancer surgery. Revista Latino-Americana De Enfermagem, seria online (1):35-43.
- Petito, E. L., Esteves, M. T., Elias, S., Facina, G., Nazário, A. P., & Gutiérrez, M. R. (2014). The influence of the initiation of an exercise programme on seroma formation and dehiscence following breast cancer surgery. Journal Of Clinical Nursing, 23(21/22), 3087-3094, doi:10.1111/jocn.12544.
- Pierce, J.P., Stefanick, M.L., Flatt, S.W., Natarajan, L., Sternfeld, B., Madlensky, L. & Rock, C.L. (2007). Greater survival after breast cancer in physically active women with high vegetablefruit intake regardless of obesity. Journal of Clinical Oncology, 25, 2345–2351, doi: 10.1200/JCO.2006.08.6819.
- Pinto e Silva, M.P., Derehain, S.F.M., Rezende, L., Cabello, C. & Martinez EZ. (2004). Movimento do ombro após eirurgia por eareinoma invasor da mama: estudo randomizado prospeetivo eontrolado de exereíeios livres versus limitados a 90° no pós-operatório. RBGO, 26(2):125-30.
- Puscas, D., Zamora, E. & Vlad, C. (2013). Recuperarea prin exercițiu fizic și masaj în cancerul de sân operat. Ed. Risoprint, 47-51.
- Schwartz, A.L., Winters-Stone, K., & Gallucci, B. (2007). Exercise effects on bone mineral density in women with breast cancer receiving adjuvant chemotherapy. Oncology Nursing Forum, 34, 627–633, doi: 10.1188/07.ONF.627-633.
- Springer, B.A., Levy, E., McGarvey, C., Pfalzer, L.A., Stout, N.L. & Gerbeer, L.H. (2010). Pre-operative assessment enables early diagnosis and reeovery of shoulder funetion in patients with breast eaneer. Breast Caneer Res Treat, 120:135-47.
- Thakur, R.R., Anjali, B., Amrit, K. (2016). Effectiveness of early physiotherapy to prevent lymphedema after breast cancer related surgery. Ind J Physiother Occupat Ther, 10(3):96-101, doi:10.5958/0973-5674.2016.00089.7.
- Torres, L.M., Yuste Sanchez, M.J., Zapico, G.A., Prieto, M.D., Mayoral del Moral, O., Cerezo, Tellez E. & Minayo Mogollon, E. (2010). Effectiveness of early physiotherapy to prevent lymphoedema after surgery for breast cancer: Randomised, single blinded, clinical trial. British Medical Journal, 340: b5396.
- Travier, N., Velthuis, M. J., Steins Bisschop, C. N., van den Buijs, B., Monninkhof, E. M., Backx, F., & May, A. M. (2015). Effects of an 18-week exercise programme started early during breast cancer treatment: a randomised controlled trial. BMC Medicine, 13(1), 1-11, doi:
- Valenti, M., Porzio, G. & Aielli, F. (2008). Physical exercise and quality of life in breast cancer survivors. Int J Med Sci, 5:24–28.
- Visovsky, C. (2006). Muscle strength, body composition, and physical activity in women receiving chemotherapy for breast cancer. Integr Cancer Ther, 5:183–191.
- Voskuil, D. W., van Nes, J. H., Junggeburt, J. C., van de Velde, C. H., van Leeuwen, F. E. & de Haes, J. M. (2010). Maintenance of physical activity and body weight in relation to subsequent quality of life in postmenopausal breast cancer patients. Annals Of Oncology, 21(10), 2094-2101.
Copyright information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
About this article
Publication Date
25 May 2017
Article Doi
eBook ISBN
978-1-80296-022-8
Publisher
Future Academy
Volume
23
Print ISBN (optional)
-
Edition Number
1st Edition
Pages
1-2032
Subjects
Educational strategies, educational policy, organization of education, management of education, teacher, teacher training
Cite this article as:
Pușcaș, D., Achimaș- Cadariu, P., Vlad, C., Moț, A., & Tache, S. (2017). Physiotherapy Following Breast Surgery For Cancer. In E. Soare, & C. Langa (Eds.), Education Facing Contemporary World Issues, vol 23. European Proceedings of Social and Behavioural Sciences (pp. 1648-1660). Future Academy. https://doi.org/10.15405/epsbs.2017.05.02.202

