Abstract
Nowadays biomedical industry and biotechnology are one of the main directions of the modern post-industrial economy, but their condition in the Russian Federation has controversial nature. On the one hand, Russia is lagging behind leading countries in terms of production of biotechnologies and products of the biomedical industry and growth rates, and on the other hand, it is characterized by consumer demand for such products. This study examined the process of the management of innovations in the field of biomedicine, identified the general directions for the development of biomedical industry as a strategic priority of the national economy. Nowadays, a public-private partnership should occupy a significant place among the tools for the innovative development of biomedical industry. One of the main directions of the state development strategy is the provision of socio-economic conditions and information platform for the effective implementation of the adopted state industrial strategies. This fully applies to innovative development. Therefore, it is necessary to pay due attention to management tools in order to ensure the effectiveness of the implementation of the Strategy aimed at the stimulation of research and development in biomedical industry. The article examines the phenomenon of public-private partnership as a tool for solving complex problems in socially significant areas, including biomedicine. The features of innovation management based on public-private partnership in the field of biomedicine are considered. The tools for innovation management activities are identified. The examples of the world practice of public-private partnership in biomedical industry are analyzed.
Keywords: Innovationbiomedicinehealthcarepublic-private partnership
Introduction
Modern biomedicine is actively and dynamically developing. Its rapid improvement puts this field of activity at the forefront of global science and new innovative trends. Undoubtedly, this is directly related to social aspect of the industry itself. Innovations in biomedicine increasingly affect the quality of life of the population.
In the general paradigm of innovative development of the government, medical projects fall into the category of innovative medical technologies (in particular, the promising area of biomedicine). In this regard, it seems relevant to consider the system of management of innovations in the field of biomedicine related to the specificity of products and its high added value.
Problem Statement
In modern science, scientists focus their attention not only on the solution of tactical tasks of state economic development, but also on the development and justification of strategic actions, including the management of innovations in the biomedical field. Innovative theories in the dynamic and evolutionary aspects were developed by Schumpeter (1982), Drucker (2003), Mensch (1979), Perez (2009), Hirooka (2006) and others. The category “innovation” is characterized by universality, a wide scope of application and complexity of structural elements that have many approaches to the disclosure of its content. It is innovations, according to the point of view of Schumpeter (1982), that are the “stem of a new type of competition” and give rise to long business cycles. Scientists developed an innovative theory of long waves, which was later integrated into the general innovation theory of economic development.
Drucker (2003) considers innovation as a socio-economic concept, identifying increase of the return on invested resources as a purpose of an innovative solution. Hirooka (2006) substantiated the process of clustering of innovations and their synergistic effect, causing a significant integrated growth of the economy and contributing to the revitalization of its development. In addition, Hirooka (2006) defined the innovation paradigm as a combination of three logistic trajectories with a cascade structure: technological (development of key technology), design and development (creation and commercialization) and diffusion (output to the market, distribution to the moment of saturation).
Innovations in biomedicine are modern technologies for the creation and use of pharmaceutical and diagnostic tools, instruments, or methods with a high level of competitiveness. Usually, the incentive to start an innovation project is a scientific discovery or achievement.
In his study, Tylecote (2019) notes that the feasibility paradigm covers a combination of technically and economically interconnected innovations and affects most industries, including biotechnology. The author considers in what sense biotechnology has reached technological maturity, and what “inconsistencies” between it and the socio-institutional structure interrupt its further development and dissemination. It is argued that biotechnology is necessary in order to play a key role in combating climate change, which, in turn, may lead to the unfolding of its potential.
The authors Gartland and Gartland (2018) in their research write that strategies for biotechnology should take into account opportunities for research, innovation and business growth. At the regional level, the cooperation between public and private sectors provides the potential for such growth and the creation of centers of competence. When considering recent advances in biotechnology, opportunities for intellectual, strategic and specialized investments are discussed. Successful centers of competence should encourage public-private partnerships, clustering and global collaboration based on best practices, sound strategies and innovations if they want to remain sustainable for a long period of time.
Innovative activity and development of innovations have their own management peculiarities. In any sphere, including biomedicine, enterprises need a developed infrastructure. In medical organizations, this means innovative equipment, creation of the conditions for introducing innovations, which are priority aspects of the development of new technologies (Pugachev, 2013).
In the biomedical industry, innovations provide not only the development of molecular and regenerative medicine, targeted therapy, biochipping and neuroinformatics, but also the efficiency of resource potential use, the introduction of new forms and tools for medical care provision, the formation of mechanisms for new technologies management.
The objective prerequisites for the development of innovative potential of the biomedical industry are as follows:
- Public importance of the health care, health and quality of life of the population, the national security of the state;
- Expansion of the scope of research and development and sources of funding;
- Increase in the attractiveness of investments in the field of medical innovation (Dmitrik, 2017).
Innovative activity in the field of biomedicine is aimed at the creation of new breakthrough biotechnologies, at the practical use of scientific advances and advanced medical experience, and the development of new pharmaceutical products. One of the methods of development and management of innovations in the field of biomedicine is considered to be a public-private partnership (Figure
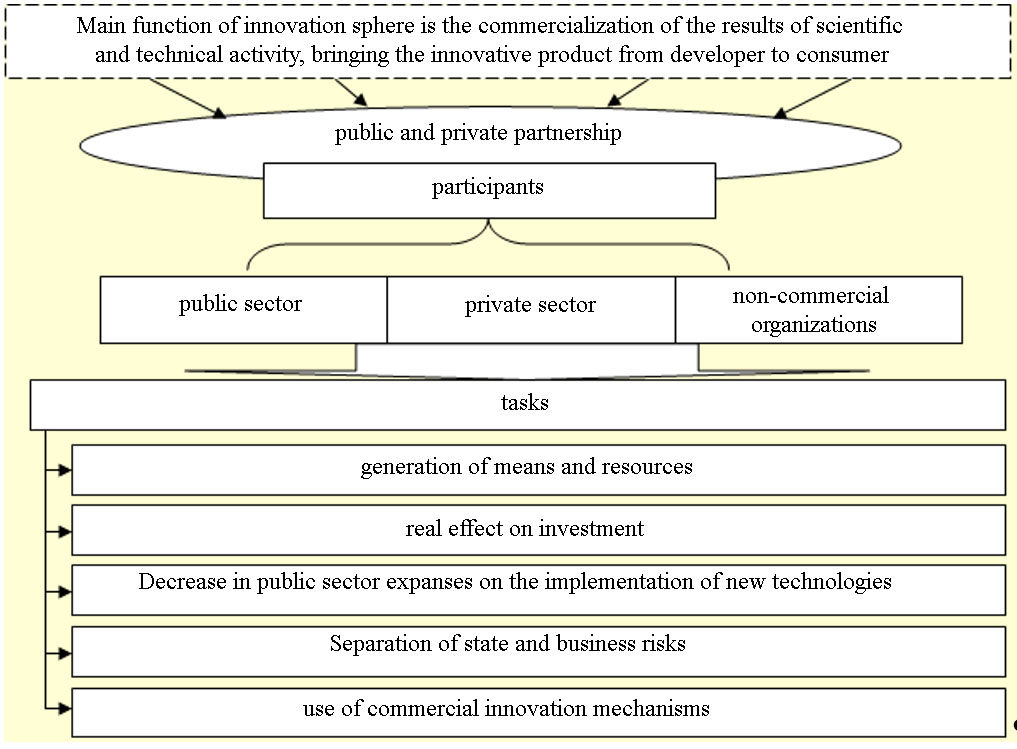
Public-private partnerships are an effective way to take advantage of the capabilities of public and private sectors in order to solve health problems that neither side can solve properly. Each side makes a contribution. Public sector, for its part, can provide support for research and participate in funding. Pharmaceutical companies can provide the organization of the process from drug development to regulatory evaluation and market launch.
In scientific publications much attention is paid to the study of the role of public-private partnership in the development of economic sectors.
The research by Cui, Wang, Liu, and Coffey (2019) shows that the better the overall collaborative environment, the better the capabilities and characteristics of the participants and the cooperation between public and private sectors is more effective. In addition, the level of cooperation has a direct positive effect on cost and efficiency. A stable macroeconomic state has the most significant impact on the terms of cooperation in the framework of public private partnerships.
Hou (2017), examining the current situation and problems, gives a current assessment of the price-quality ratio in a Chinese-state partnership project. The author notes that one of the promising areas of reforming the Chinese economy is the active introduction of a public-private partnership in the support system for various economic sectors.
Almarri and Boussabaine (2017) in their study note that governments are increasingly entering into partnerships with the private sector in a public-private partnership model for the development of government projects. Their study shows that government guarantees, macroeconomic conditions, general powers between the public and private sectors, social support and a transparent procurement process have a positive effect on the analysis of the effectiveness of the use of finances.
The researchers Bao, Chan, Chen, and Darko (2018) in their study also note that the process of the development of public-private partnerships is complex and dynamic throughout life project cycle: the stages of identification, preparation, procurement, implementation, transfer and post-transfer of the project. This is certainly applicable to the management of innovations in the field of biomedicine. The authors note that different phases of public-private partnerships face various problems, attracting the unequal attention of researchers. The results of this study show the relationship between important project issues and the corresponding phases of public-private partnership, which can serve as a valuable guide for partners in the development of effective project management strategies.
Taking into account the complexity and broad interests of stakeholders in public-private partnership projects, different parties have different expectations and determine the success of a partnership project. Osei-Kyei and Chan (2018) discusses differences in the perception of success criteria for public-private partnership projects among stakeholders. The results of the research of the authors show that each stakeholder group considers effective risk management as the most important criterion for success.
Research Questions
The authors of the article consider the role of public-private partnership in the management of innovations in the field of biomedicine.
Purpose of the Study
The purpose of this article is to explore the process of the management of innovations in the field of biomedicine with the application of the global best practices of interaction between the state and business of public-private partnerships, as well as to determine the general directions of development of the biomedical industry as a strategic priority of national economy.
Research Methods
The methodological basis of the research is formed by general scientific methods, system-structural and functional approaches, modern analytical methods and technologies. The provisions of the study are justified by particular scientific methods (formal, comparative, functional, specification, etc.).
Findings
In recent years, Russia there is quite active creation of public-private partnership tools in the innovation sphere: the creation of technology-innovation zones, technology parks, business incubators, technology platforms, participation in innovative development of biomedicine RUSNANO, the Investment Fund, etc.
The main advantages of public-private partnerships are:
- Provision of competitiveness and implementation of innovative projects on the principle of partnership;
- Discounting investments (return on investment at the expense of future cash flows);
- Provision of qualitative functioning of the organizational system of relations between partners with the distribution of powers, obligations and limits of responsibility;
- Identification of growth factors, areas of project implementation, external threats and internal risks, their analysis, assessment, formation of a management system.
The process of the management of innovations based on public-private partnership in the field of biomedicine includes a certain sequence of steps presented in Figure
Public-private partnerships should occupy a significant place among the tools for innovative development of the biomedical industry, in particular, in the development of innovations related to the treatment of chronic and infectious diseases, the development of popular medical products, the creation of information mechanisms for foresight, cognitive and multicenter research, assistance in the framework of international partnerships, etc.
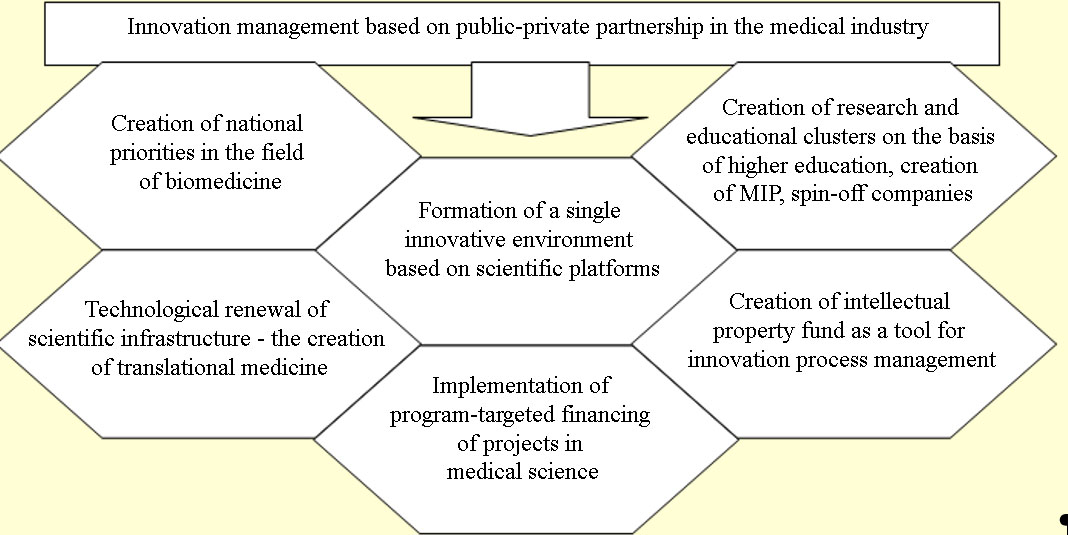
Research and development in biomedicine is conducted mainly by non-state pharmaceutical and biotechnological companies (Pugachev, 2013). At the same time, the decision to create an appropriate innovative product is made on the basis of an analysis of the return on investment and its profitability in the future. Since any innovative project is associated with significant financial risks and are long-term in the field of biomedicine investments (the development and introduction of drug on the market can take 12-17), it is necessary to involve state, educational and scientific organizations in such projects. At the project stage of the creation of innovation, state participation in financing reaches about 50% and subsequently, with promising predictions of drug testing, it decreases (Khaitov, Alekseev, & Trofimov, 2017).
The examples of the use of public-private partnership in the field of scientific research and research in the European Union, can be presented by the Innovative Medical Initiative, the budget of which for the period from 2014 to 2024 amounts to 3.3 billion Euros, and the goal is to integrate science, education, government and business to accelerate the creation of new medical products. As part of this public-private partnership, financial risks are shared between the Horizon-2020 program of European Union, funds of the European Federation of Pharmaceutical Manufacturers Associations members and associated partners, while the contribution of last is non-monetary in nature (they provide mostly resources or equipment) (Dmitrik, 2017).
Public-private partnership in biomedicine is also actively used in the United States: the unification of the National Institutes of Health, the Food and Drug Administration and pharmaceutical companies, which create, for example, drugs for Alzheimer disease, develop technologies in the field of genomics, neuroinformatics, create an information system for data exchange of genetic and phenotypic studies, etc.
Conclusion
Biomedicine is the industry where innovation is the most important source of competitiveness. Usually innovative projects are implemented through the formation of public-private partnerships. This approach offers a number of advantages – integration of resources, knowledge and experience, financial risks sharing, which allows conducting large-scale research, as well as directing efforts in accordance with the structure of the morbidity of population, that is, the development of drugs that meet real medical needs.
The development of public-private partnerships contributes to increasing transparency based on the integration of resources, the exchange of data, knowledge and experience and the creation of information infrastructure. The use of public-private partnership in Russia may be appropriate from the point of view of modernization in the field of biomedicine, improving the quality of medical services and access to medicines. The important role in the development of public-private partnership is the increase in the innovation and investment attractiveness of biomedicine for international investors and organizations.
Acknowledgments
The research was conducted as a part the project the state task in the field of scientific activity in accordance with project No. 26.2758.2017 / 4.6 for 2017-2019 on “System of analysis of formation and distribution of innovative products expenses based on the infrastructure concept”.
References
- Almarri, K., & Boussabaine, H. (2017). The Influence of Critical Success Factors on Value for Money Viability Analysis in Public–Private Partnership Projects. Project Management Journal, 48(4), 93–106.
- Bao, F., Chan, A. P., Chen, C., & Darko, A. (2018). Review of public-private partnership literature from a project lifecycle perspective. Journal of Infrastructure Systems, 24(3), 04018008.
- Cui, C., Wang, J., Liu, Y., Coffey, V. (2019). Relationships among Value-for-Money Drivers of Public-Private Partnership Infrastructure Projects. Journal of Infrastructure Systems, 25(2), 04019007.
- Dmitrik, E. N. (2017). Public-private partnership in healthcare. International experience. Retrieved from: https://www.apteka.ua/article/423444
- Drucker, P. F. (2003). Tasks of management in the XXI century: рer. from English. Moscow: Williams.
- Gartland, K. M. A., & Gartland, J. S. (2018). Opportunities in biotechnology. Journal of Biotechnology, 282, 38–45.
- Hirooka, M. (2006). Innovation Dynamism and Economic Growth. A Nonlinear Perspective. Cheltenham, UK; Northampton, MA:.
- Hou, S. (2017). Value-for-Money assessment in China's public-private-parternship project present situation, problems and policy suggestions. In 4th International Conference on Industrial Economics System and Industrial Security Engineering. IEIS, 8078650.
- Khaitov, R. M., Alekseev, L. P., & Trofimov, D. Yu. (2017). Public-private partnership as a basis for innovation in high-tech biomedical research. Retrieved from: http://yandex.ru/clck/jsredir?bu
- Mensch, G. (1979). Stalemate in Technology – Innovations Overcome the Depression. New York: Ballinger Publishing Company.
- Osei-Kyei, R., Chan, A. P. C. (2018). Stakeholders’ perspectives on the success criteria for public-private partnership projects. International Journal of Strategic Property Management, 22(2), 131–142.
- Perez, C. (2009). Technological revolutions and techno-economic paradigm. Tallinn: Tallinn University of Technology. Retrieved from: http://e-tcs.org/wp-content/uploads/2012/04/PEREZ-Carlota-Technological-revolutions-and-techno-economic-paradigms1.pdf
- Pugachev, N. S. (2013). Public-private partnership in the innovation sphere. In Proceedings of the II International Scientific Conference “Actual Issues of Economics and Management” (pр. 52–54). Moscow: Buki-Vedi. Retrieved from: https://moluch.ru/conf/econ/archive/91/4249/
- Schumpeter, Y. (1982). The Theory of Economic Development: Research on entrepreneurial profit, capital, credit, and business cycle. Moscow: Progress.
- Tylecote, A. (2019). Biotechnology as a new techno-economic paradigm that will help drive the world economy and mitigate climate change. Research Policy, 48(4), 858–868.
Copyright information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
About this article
Publication Date
28 December 2019
Article Doi
eBook ISBN
978-1-80296-075-4
Publisher
Future Academy
Volume
76
Print ISBN (optional)
-
Edition Number
1st Edition
Pages
1-3763
Subjects
Sociolinguistics, linguistics, semantics, discourse analysis, science, technology, society
Cite this article as:
Musostova*, D., Popova, L., Korostelkina, I., Dedkova, E., & Arzamasceva, N. (2019). Public-Private Partnership As A Tool For Innovation Management In Biomedicine. In D. Karim-Sultanovich Bataev, S. Aidievich Gapurov, A. Dogievich Osmaev, V. Khumaidovich Akaev, L. Musaevna Idigova, M. Rukmanovich Ovhadov, A. Ruslanovich Salgiriev, & M. Muslamovna Betilmerzaeva (Eds.), Social and Cultural Transformations in the Context of Modern Globalism, vol 76. European Proceedings of Social and Behavioural Sciences (pp. 2366-2372). Future Academy. https://doi.org/10.15405/epsbs.2019.12.04.316
