Abstract
A good quality of life includes a good equilibrium while standing and walking, performing daily activities, without any risk of falling. Inner ear has a large influence on steadiness and any pathology in its normal functioning induces dizziness and gait disturbances. This paper presents results from a clinical study in which we treated gait problems through bimodal treatment: medical treatment with Betahistine and virtual reality-based vestibular rehabilitation. 36 patients with vestibular neuritis who presented at the Institute of Phono-Audiology and ENT Functional Surgery from Bucharest, Romania, were included in the study. They were evaluated at the admission and after 6 and 12 weeks of bimodal treatment. Evaluation was performed both subjectively and objectively. Questionnaires were used for subjective evaluation by the patient him/herself (Dizziness Handicap Inventory – DHI, Activities-specific Balance Confidence Scale – ABC Scale) and by the ENT Physician (Berg Balance Scale – BBS). Gait disturbances were measured with specific tests (Computerised Dynamic Posturography – CDP, Dynamic Gait Index – DGI and Tinetti Performance-Oriented Mobility Assessment – POMA). Results showed quick normalisation of physical performance for simple activities on stable and smooth surfaces and good lightening environment. Early vestibular rehabilitation induced better and quicker compensation of the vestibular impairment. Using virtual reality as a vestibular rehabilitation method increased patient’s adherence and compliance to the long-term treatment.
Keywords: Vestibular impairmentgait disturbancesvestibular rehabilitation program
Introduction
Dizziness as an overall pathological condition is very frequently met in general population – it has a 7.7 per 1000 patients/year incidence (Van der Linden, Westert, De Bakker, & Schellevis, 2004), with a life time prevalence of 29.3%. It can be the result of different and various conditions, vestibular, neurological, cardiovascular, endocrinological or psychological ones, and finding the disturbed system is mandatory in healing the patient.
Dizziness induced by vestibular lesions has a negative impact on health-related quality of life, especially due to physical and functional limitations in daily activities and at work. Complaints are severe and with high impact on patient’s quality of life: 70% of these patients seek for medical help, 41% are unable to work at the onset of the vestibular lesion, 40% are not able to perform daily activities and 19% are home-restricted. (Courjon, Flandrin, Jeannerod, & Schmid, 1982)
Problem Statement
When a vestibular lesion occurs, main complaints address vertigo, unsteadiness and gait disturbances. Gait and postural control disturbances impede upon individual mobility and patient’s functional independence. This pathological condition has an additional negative aspect due to high- growing population of elderly – if vestibular lesion occurs in adult life, the period they have to deal with its sequelae is longer and additional equilibrium problems will occur (orthopaedic, sight disturbance, neurological ones); if vestibular lesion occurs in elderly life, healing will be much more difficult due to their low base-line equilibrium before the vestibular lesion – age-related orthopaedic and visual problems with already a high risk of falling.
There are two major categories of gait disturbances: continuous and episodic (one single episode or recurrent ones), a characteristic important to know when assessing functional deficit, treatment protocol and long-term prognostic.
Vestibular system has an important role in equilibrium, together with vision and somatosensory system. Any permanent vestibular lesion will induce severe vertigo, dizziness and/or disequilibrium. Vestibular neuritis is the most common type of unilateral vestibular loss. It is, of course, less severe than a permanent central vestibular lesion, because a peripheral vestibular deficit has the advantage to be overcome in time by the central vestibular compensation process.
Research Questions
At the onset of vestibular neuritis due to the large (even all) number of affected vestibular nerve fibres, patients experience severe vertigo, impossibility of stance and walk alone. It is an example of continuous gait and balance impairment, because peripheral lesion is never recovered, but compensated by the vestibular central structure neuroplasticity through habituation, adaptation and sensory substitution.
Thus, the patient will regain balance in predictable situations by changes in his/her motor behaviour.
The present research aims to answer the question: “Are vestibular rehabilitation (VR) programs beneficial for recovering gait disturbances induced by vestibular neuritis?”
Purpose of the Study
The purpose of this study is to evaluate and quantify the initial vestibular deficit, as degree of lesion and impact on patient’s quality of life through bed-side examination, vestibular test and specific questionnaires. These methods also allow us to monitor the benefit of the recommended treatment protocol.
Research Methods
36 patients (16-57 years old) who successively presented at our Institute for severe vertigo and imbalance, diagnosed with vestibular neuritis in acute stage, were included in this study. Initial evaluation included history, bed-side otoneurologic evaluation (binocular infra-red camera system) and pure tone audiometry.
All of them were subjected to the bimodal treatment program, based on Betahistine and customised vestibular rehabilitation (VR) exercises, immediately after a three-day course of acute stage. Before and one month after treatment, patients filled in the Dizziness Handicap Inventory (DHI) and Activities-specific Balance Confidence Scale (ABC Scale) questionnaires to have a self-report of their disequilibrium condition.
Besides bed-side oto-neurological clinical examination, patients were further evaluated with Berg Balance Scale questionnaire and other physical tests – Computerised Dynamic Posturography (CDP) with SMART balance device from Neurocom Company, Dynamic Gait Index (DGI), Berg Balance Scale, Short Physical Performance Battery (SPPB) and Performance-Oriented Mobility Assessment (POMA), for the objective quantification of gait disturbances.
Findings
Initial evaluation showed spontaneous nystagmus in 67% of patients and pathological scores in all physical tests, with moderate risk of falling. In general, patients self-reported a moderate decrease in health-related quality of life.
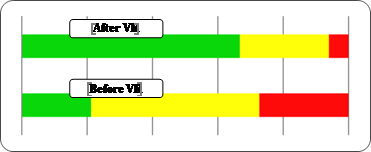
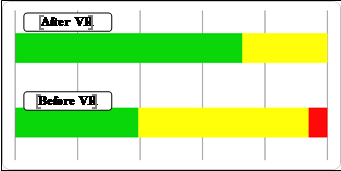
After one month of bimodal treatment, results improved in all physical tests and the majority of patients reported significant improvement in quality of life (Figures
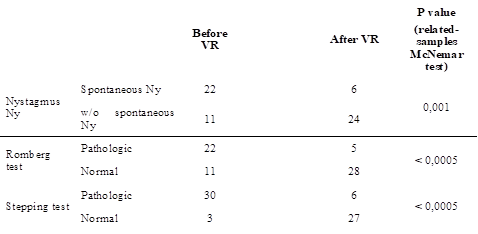
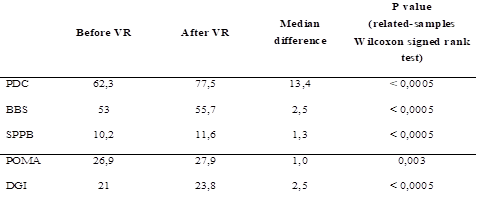
Acute unilateral vestibular loss (UVL) induced by vestibular neuritis leads to huge insecurity while standing or walking, with a high propensity to fall in the initial stage (first three weeks) due to massive asymmetry between inner ear vestibular sensory structures. This disability is the result of massive imbalance in physiological inhibitory-excitatory push-pull action upon axial muscular fibres in the balance process.
Recovery after UVL implies neuroplasticity phenomena at the central vestibular structure level (vestibular nuclei, cerebellum and thalamus) in the first month after the lesion onset, known as central vestibular compensation (Darlington & Smith, 2000; Kitahara, Takeda, Kiyama, & Kubo, 1998; Newlands & Perachio, 1990). The process is long-lasting (over three month), imperfect and incomplete (high-acceleration or velocity head movements are not always compensated), but enables these patients to regain daily comfort.
In order to facilitate and enhance this natural healing process, appropriate treatment should start immediately after the acute stage, ideally three days after the UVL onset. The patient must not lay in bed neck-stiffed, even though the slightest movements provoke vertigo and imbalance. The first month after the UVL onset is an opportunity window for the neuroplasticity process. It must be maximised, first by a short period (three days) of vestibular suppressant drugs and second by stimulating central vestibular compensation with appropriate drugs and VR exercises. (Courjon et al., 1982; de Waele, Vidal, Tran Ba Huy, & Freyss, 1990; Georgescu & Stoian, 2011; Georgescu, Stoian, Mogoanță, & Ciubotaru, 2012; Georgescu, 2017; Halmagyi & Curthoys, 1996; Herdman, 2014)
The aim of central vestibular compensation is to regain symmetry in the resting discharge of vestibular nuclei (Hall et al. 2016; Hillier & Hollohan, 2007) by:
Opening unused vestibular synapses on the lesion side;
New sprouting in the peripheral vestibular structures ipsilateral to UVL;
Slowing down the ipsilateral inhibitory cerebellar activity and activation of the vestibular-hypothalamic-vestibular loop;
Inhibition of the resting discharge rate in the contralateral medial vestibular nucleus through high cerebellar inhibitory signals.
Physiological neurotransmitter in the central vestibular structures (mainly thalamus) is Histamine. For this reason, Betahistine, a H3 receptor antagonist, is the first-choice drug treatment to enhance vestibular compensation process. Betahistine enhances release of the neurotransmitter in the vestibular pathway in order to inhibit activity in the contralateral healthy vestibular system and to stimulate nerve sprouting and the opening of closed peripheral vestibular synapses in the ipsilateral vestibular system (Figure
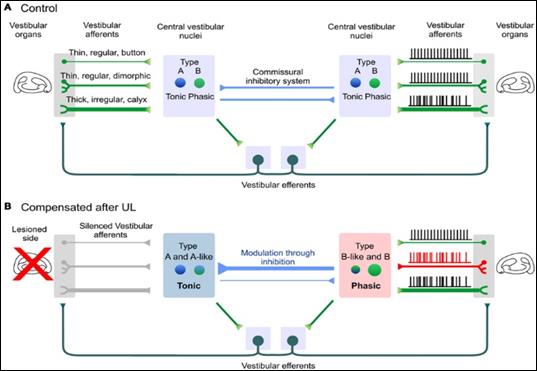
which is very useful for recovery after UVL (Lacour & Sterkers, 2001).
For patients included in our study, results after one month of vestibular rehabilitation through customised programs showed significant improvement in self-reported questionnaires, as well as in medical evaluation (questionnaire and physical test):
Total and subcategory DHI scores showed a statistically significant improvement (related-samples Wilcoxon signed-rank test, p < 0.0005).
Functional level indicated by ABC Scale significantly improved (related-samples Wilcoxon signed-rank test, p < 0.0005), 72.7% of patients having a high functionality and 28.3% a moderate one. No patient experienced anymore reduced functionality.
26 patients (81.3%) normalised their CDP scores, and improvement of CDP score was statistically significant (related-samples Wilcoxon signed-rank test, p < 0.0005).
Berg Balance Scale showed normal results in 26 patients (81.2%), with a significant difference between initial and after VR evaluation (related-samples McNemar test, p < 0.0005).
SPPB score varied between 9 and 12, with a median of 11.6 ± 0.7, and improved significantly from the initial evaluation (related-samples Wilcoxon signed-rank test, p < 0.0005). Additionally, the number of patients with maximum score was significantly larger after VR (related-samples McNemar test, p < 0.0005).
POMA test showed maximum score in 30 patients (93.7%), which was a number significantly larger than the number of patients with maximum score before VR (related-samples McNemar test, p = 0.004). Improvement was significant (related-samples Wilcoxon signed-rank test, p = 0.003).
Improvement of DGI score was also statistically significant after one month of VR (related-samples Wilcoxon signed-rank test, p < 0.0005), and maximum score was present in 27 patients (84.3%), a significantly larger number than the initial one (related-samples McNemar test, p < 0.0005).
Duration of VR treatment negatively correlated with initial pathological SOT score, low scores in POMA and DGI, as well as with DHI score above 50 and ABC score over 70%. (Darlington & Smith, 2000; Georgescu et al. 2012; Georgescu, 2017; Halmagyi & Curthoys, 1996; Kitahara et al., 1998)
Conclusion
Walking is a common daily activity, but also a complex one. Normal and safe walking implies intact cognition, command and execution. Gait impairment reduces individual motor independence, increases the risk of falling and reduces the quality of life.
Peripheral vestibular lesions induce a sudden loss in stance and gait control, with secondary long-term dysfunctionality in equilibrium, essential for sudden movements and busy visual environments. Early specific treatment protocols enhance physiological recovery after UVL through the central vestibular compensation process and allow faster and better overcoming of the initial gait impairment.
Vestibular rehabilitation plays an important role in the recovery of the patient with vestibular neuritis. Properly conducted, rehabilitation exercises help these patients return almost completely to their normal state of health and daily activity. The sooner this treatment starts, the faster the recovery is. Weekly sessions of virtual reality methods showed better results than at-home daily exercises alone.
Duration of VR treatment negatively correlated with initial pathological SOT score, low scores in POMA and DGI, as well as with DHI score above 50 and ABC score over 70%.
References
- Courjon, J. H., Flandrin, J. M., Jeannerod, M., & Schmid, R. (1982). The role of the flocculus in vestibular compensation after hemilabyrinthectomy. Brain Research, 239(1), 251-257.
- Darlington, C. L., & Smith, P. F. (2000). Molecular mechanisms of recovery from vestibular damage in mammals: Recent advances. Progress in Neurobiology, 62(3), 313-325.
- de Waele, C., Vidal, P. P., Tran Ba Huy, P., & Freyss, G. (1990). Vestibular compensation. Review of the literature and clinical applications. Annales d’Oto-Laryngologie et de Chirurgie Cervico-Faciale, 107(5), 285-298.
- Georgescu, M. (2017). Vestibular rehabilitation – Recommended treatment for permanent unilateral vestibular loss. International Journal of Neurorehabilitation, 4: 4. Retrieved from https://www.omicsonline.org/open-access/vestibular-rehabilitation--recommended-treatment-for-permanentunilateral-vestibular-loss-2376-0281-1000282.pdf
- Georgescu, M., & Stoian, S. (2011). Neuronita vestibulară în sarcină – Prezentare de caz. Ginecologia, 7(23), 58-61.
- Georgescu, M., Stoian, S., Mogoanță, C. A., & Ciubotaru, Gh. V. (2012). Vestibular rehabilitation – Election treatment method for compensating vestibular impairment. Romanian Journal of Morphology and Embryology, 53(3), 651-656.
- Hall, C. D., Herdman, S. J., Whitney, S. L., Cass, S. P., Clendaniel, R. A., Fife, T. D., … Woodhouse, S. N. (2016). Vestibular rehabilitation for peripheral vestibular hypofunction: An evidence-based clinical practice guideline. Journal of Neurologic Physical Therapy, 40(20), 124-155.
- Halmagyi, M., & Curthoys, I. (1996). How does the brain compensate for vestibular lesions? In: R. W. Baloh & G. M. Halmagyi (Eds.), Disorders of the vestibular system (pp. 145-151). New York: Oxford University Press.
- Herdman, J. S. (Ed.). (2014). Vestibular rehabilitation (4th ed.). Philadelphia, USA: F. A. Davis Co.
- Hillier, S. L., & Hollohan, V. (2007). Vestibular rehabilitation for unilateral peripheral vestibular dysfunction. Cochrane Database Systematic Review, 4. Retrieved from https://doi.org/10.1002/14651858.CD005397.pub4
- Kitahara, T., Takeda, N., Kiyama, H., & Kubo, T. (1998). Molecular mechanisms of vestibular compensation in the central vestibular system – Review. Acta Oto-Laryngologica (Supplement), 539, 19-27.
- Lacour, M. (1989). Vestibular compensation: Facts, theories and clinical perspectives. Amsterdam: Elsevier.
- Lacour, M., & Sterkers, O. (2001). Histamine and Betahistine in the treatment of vertigo: Elucidation of mechanisms of action. CNS Drugs, 15(11), 853-870.
- Newlands, S. D., & Perachio, A. A. (1990). Compensation of horizontal canal related activity in the medial vestibular nucleus following unilateral labyrinth ablation in the decerebrate gerbil. II. Type II neurons. Experimental Brain Research, 82(2), 373-383.
- Pfaltz, C. R., & Kamath, R. (1970). Central compensation of vestibular dysfunction. I. Peripheral lesions. Practica Oto-Rhino-Laryngologica, 32, 335-349.
- Van der Linden, M. W., Westert, G. P., De Bakker D. H., & Schellevis, F. G. (2004). Tweede nationale studie naar ziekten en verrichtingen in de huisartspraktijk. Klachten en aandoeningen in de bevolking en in de huisartspraktijk. Utrecht/Bilthoven: NIVEL/RIVM. Retrieved from https://www.nivel.nl/sites/default/files/bestanden/ns2_r1_h00.pdf
- Zee, D. S. (1994). Vestibular adaptation. In D. S. Herdman (Ed.), Vestibular rehabilitation (3rd ed.) (pp. 77-90). Philadelphia: F. A. Davis Co.
Copyright information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
About this article
Publication Date
16 February 2019
Article Doi
eBook ISBN
978-1-80296-054-9
Publisher
Future Academy
Volume
55
Print ISBN (optional)
-
Edition Number
1st Edition
Pages
1-752
Subjects
Sports, sport science, physical education
Cite this article as:
Georgescu, M., & Cernea, M. (2019). Gait Disturbance Rehabilitation Through Virtual Reality. In V. Grigore, M. Stănescu, M. Stoicescu, & L. Popescu (Eds.), Education and Sports Science in the 21st Century, vol 55. European Proceedings of Social and Behavioural Sciences (pp. 465-472). Future Academy. https://doi.org/10.15405/epsbs.2019.02.58
