Abstract
Children with pediatric cancer usually treated with chemo- and radiotherapy. Because of such treatment, psychologists and health professionals declare cognitive and motor abilities decline in cancer patients. Timing is extremely important for movement and speech, and understanding the neurobiological basis of rhythm perception and reproduction can be helpful in addressing motor and cognitive recovery after brain lesions. The aim of our study was to investigate audio-motor synchronization abilities in children with different nervous system lesions. Our sample included 98 children, divided into three groups. The first group was consisted of children treated for posterior fossa tumors, mostly medulloblastoma and astrocytoma (PFT-group, n = 44, mean age 12.09 2.92 years). Our results suggest that degree of brain damage after cancer and treatment is associated with ability to synchronize arm movements with auditory cues (PFT > ALL > control). Healthy children are able to anticipate stimuli, but ALL and especially PFT patients mostly reacted after stimulus onset.
Keywords: Childrenaudio-motor synchronizationposterior fossa tumoracute lymphoblastic leukemiarhythm
Introduction
Entrainment to sensory rhythmic stimuli is important in people’s everyday life in order to organize behavior and to time daily events. It was shown that even infants are able to perceive time and to adapt their physiological functions to different time intervals (Bobin-Bègue et al., 2014). It was also shown than even 1 month of age infants are able to time temporal intervals and there is improvement in time estimation throughout childhood (for a review, see Droit-Volet, 2013). Regular spontaneous manual tapping tempo could be observed in children as young as 2.5 years. Moreover, children could slow down their tap rhythm when the auditory stimulation became slower (Provasi & Bobin-Bègue, 2003). In addition, rhythm perception and reproduction is relevant to such functions as speech, language and memory (Jerde et al., 2011; Saito, 2001; Thomson & Goswami, 2008). To tap according to the given rhythm precisely one should maintain the duration of the inter-stimulus-intervals in working memory.
The most simple form of entrainment to a sensory (typically auditory) rhythmic stimulus involves perceiving and synchronizing movements with an isochronous beat with one level of periodicity, such as that produced by a metronome. Rhythm perception and entrainment abilities develop early in human life and have been suggested to be relevant to a range of functions, including speech and language development (Huss et al., 2011), and social bonding (Knoblich et al., 2011).
Children with pediatric cancer usually treated with chemo- and radiotherapy. Because of such treatment, psychologists and health professionals declare cognitive and motor abilities decline in oncological patients. Review of the recent researches revealed only two studies concerning time perception and sensorimotor synchronization in children with brain cancer (Droit-Volet et al., 2013; Provasi et al., 2014).
The results of the first study (Droit-Volet et al., 2013) indicated that the major cause of deficits in temporal judgments in children with cerebellar lesions was due to their inability to reproduce accurately short temporal intervals in association with low processing speed, rather than to a specific deficit in the perception of time. Provasi et al (2014) demonstrated that during the synchronization task, the children with medulloblastoma succeeded to modify the length of their inter-tap intervals (ITI) in response to an auditory rhythm, although with better success when the inter-stimuli intervals (ISI) were shorter than when they were longer than the ITIs of their own SMT. Correlational analyses revealed that children’s poorer synchronization performance was related to lower scores in neuropsychological tests assessing motor dexterity and processing speed.
Our previous study showed that performance in the audio-motor synchronization task (both accuracy and variability) was significantly poorer in children with cerebellum tumors than in controls, and that successful timing performance associated with fine spatial working memory, high processing speed and visual spatial span (Kasatkin et al., 2017).
Oncologists, neuropsychologists and other health care professionals intensively research cognitive impairments because of posterior fossa and cerebellum lesions concerned with recovery process of cancer survivors (Margelish, 2015; Schmahman, 2004). It was shown that there is a deficit in executive functions, attention, working memory, processing speed and academic performance in oncological patients (Lockwood et al., 1999). Audio-motor synchronization skills may be related to working memory processes mediated by the prefrontal cortex (Chen et al, 2008).
Timing and cognitive functions shared such brain regions as basal ganglia, cerebellum, prefrontal cortex (PFC), premotor cortex (PMC), supplementary motor area (SMA) (Konoike et al., 2015; Repp&Su, 2013).
Problem Statement
Children treated for posterior fossa tumors (PFT) and acute lymphoblastic leukemia (ALL) have mild to heavy nervous system damage because of chemo- and radiotherapy. ALL patients mostly have general and mild decline, and PFT patients have focal and mostly moderate to heavy nervous system’s problems. PFT affect cerebellar and IV ventricle region that by the way relate to rhythm production and perception. Because of pathological process itself and subsequent severe treatment, children demonstrate motor and cognitive problems (Margelish, 2015).
Research Questions
It was hypothesized that the ability to synchronize their movements with auditory cues, to maintain rhythm without external signals, to maintain basic rhythm in the presence of interfering rhythm (distractor) is associated with CNS damage degree. Children with ALL (mild impairment) would demonstrate worse audio-motor synchronization ability compared to controls, children with PFT would demonstrate the most pronounced decline in audio-motor synchronization tests (compared to controls and ALL-group).
Purpose of the Study
The aim of our study was to investigate audio-motor synchronization abilities in children with different nervous system lesions.
Research Methods
Our sample included 98 children, divided into three groups. The first group was consisted of children treated for posterior fossa tumors, mostly medulloblastoma and astrocytoma (PFT-group, n=44, mean age 12.092.92 years). These types of tumors affect cerebellar area and the IV ventricle. Such patients usually treated with surgery, chemo- and high doses of radiation on the local brain regions (brain stem and cerebellum). That is why children from this group demonstrated the most pronounced central nervous system (CNS) lesions and motor and cognitive problems. The second group was a group of children with acute lymphoblastic leukemia (ALL-group, n=16, mean age 11.943.62 years). ALL is the most prevalent pediatric cancer type, but the CNS damage mild to moderate because of small doses of radiation for the whole body. The third group was a control group of neurologically healthy children (control group, n=37, mean age 11.762.64).
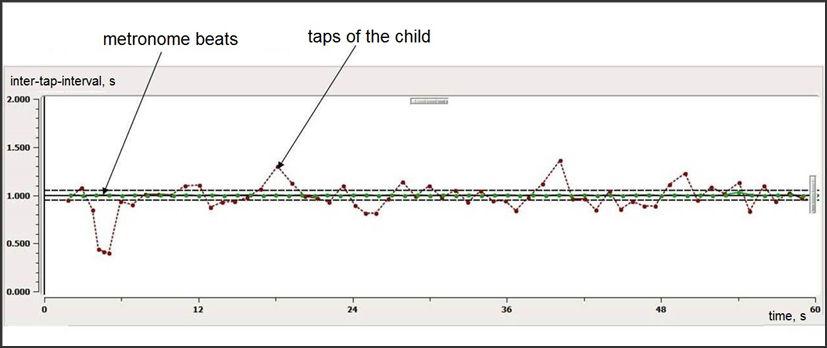
During audio-motor synchronization task children had to tap out a regular beat with their dominant hand on a special pad. Children performed four simple synchronization tasks: to synchronize hand movements (taps) with the external sounds with 40, 60, 90 and 120 bpm frequencies. The examples of the rhythmic tasks recordings are shown on Fig.
The green line indicates sound metronome beat. A child hear these sounds with regular inter-stimulus-intervals (ISI) in his/hers earphones. The red line indicates child’s taps.
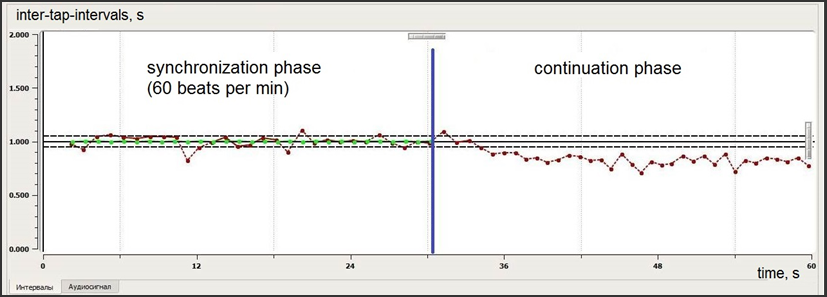
Then participant performed second (2nd) and third (3rd) tasks that are more complex. One was so-called “rhythm memory test” and the other was “interfering rhythm test” (designed for distractibility assessment) (fig. 2, 3).
The rhythm memory task consisted of two parts: in the 1st part (30 seconds) a child should tap with 60 bpm sound of metronome, then, in the 2nd part the metronome was switching off and a child continued to tap with the same rhythm without auditory cues, according to his/hers memory (next 30 seconds).
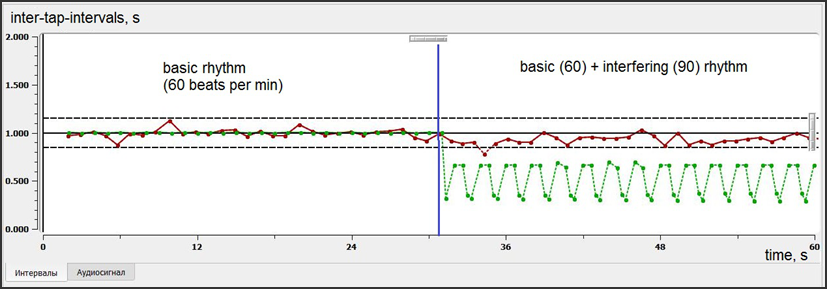
The interfering rhythm task also consisted of two parts. The 1st part was the same as in memory task: to tap accordingly to the 60 bpm metronome beat (30 seconds). During the second part additional rhythm with different frequency and sound was switched on. A child should continue to tap according to the basic 60 bpm rhythm (next 30 seconds) and not to pay attention to the interfering rhythm (distractor).
For statistical analysis of rhythm stability (consistency) and accuracy special algorithm and software was created (patent application 2018118979, TMA180157141, from 23.05.2018). Rhythm stability was assessed as a variance of inter-tap-intervals (ITI). Rhythm accuracy was assessed as a deviation of child’s ITI from the metronome’s ISI, so-called relative error. It could be either positive or negative.
Findings
Simple synchronization tests, rhythm memory test and interfering test performance was assessed as rhythm maintenance and rhythm accuracy. Rhythm maintenance we defined as ability to produce regular inter-tap-intervals. The less ITI variance (intervals do not differ much from each other), the better rhythm maintenance. Rhythm accuracy we defined as difference between child’s tap and stimulus onset (the less this difference, the more accurate is synchronization). It reflects ability to tap simultaneously with auditory cue and calculated as mean relative error, which could be either negative (a child tap before the metronome sound) or positive (a child tap after the metronome sound). The negative relative error means anticipation of the beat by child. Such situation is typical for adults and healthy children (Repp&Su, 2013).
Simple synchronization tests
The most pronounced ITI variance (poor rhythm maintenance) we can see in PFT-group during 60 bpm synchronization (fig.4, 5).
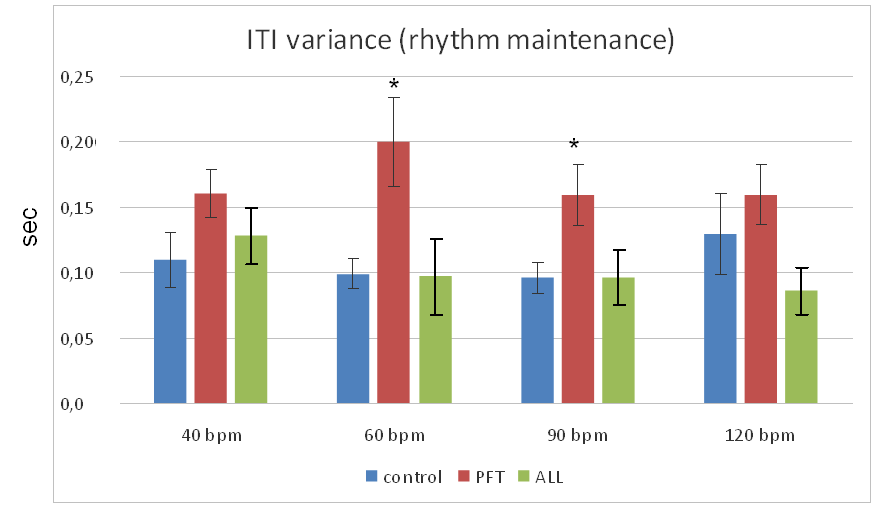
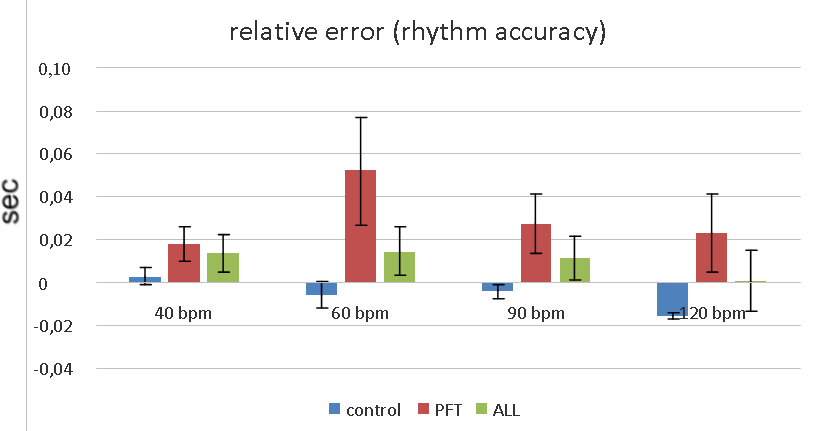
There was no significant difference between rhythm maintenance in controls and ALL-group. The later performed 120 bpm synchronization even better (non significantly) then healthy controls. PFT-groups demonstrated significantly higher ITI variance compared to controls and ALL-group in 60 bpm and 90 bpm tests according to ANOVA (F=4.19, p=0.018 and F=4.02, p=0.021 respectively).
There was no significant difference between healthy controls and ALL-group in rhythm accuracy. Hence, ALL-group demonstrated positive error in each task, and controls demonstrated negative error in all tasks except 40 bpm. Healthy children had tendency to anticipate auditory cues (negative asynchrony, or relative error), but cancer groups reacted on the sound (positive asynchrony, relative error).
Rhythm memory test
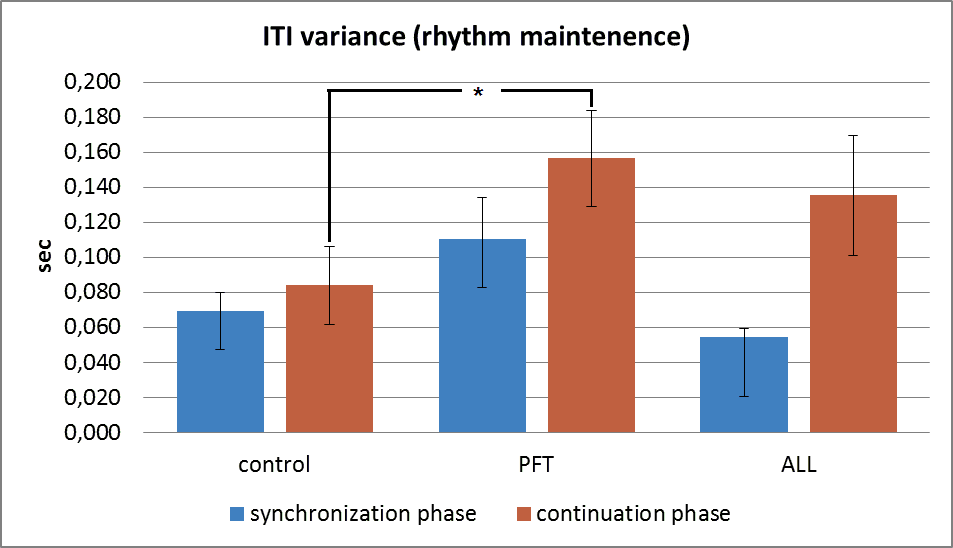
During rhythm memory test the first part (synchronization phase) is a repetition of the 60 bpm simple synchronization task. The second – continuation – phase assumes that a child have to remember the initial rhythm and to realise it without any sounds.
As we can see on fig.
Interfering rhythm test
The first part of interfering test repeats the 60 bpm synchronization test. The second part involves inhibitory mechanisms, which must help a child not to pay attention to the additional (interfering) rhythm, but to continue to tap according to the initial basic rhythm.
Fig.
The results of the study suggest that CNS lesions induced by cancer and subsequent treatment has an impact on the children’s ability to synchronize their movements with external auditory cues of different frequencies. The most affected PFT-group demonstrated the worst results in all synchronization tests. ALL-group had better results than PFT-group did.
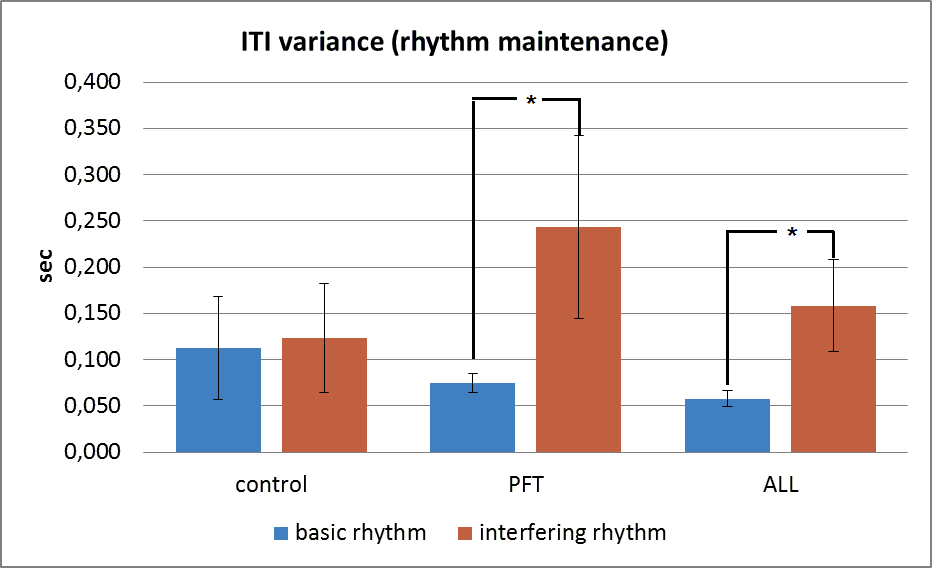
Additional cognitive load in memory test and interfering rhythm test involve higher cortical functions (executive functions). The ability for audio-motor synchronization may be related to working memory processes mediated by the prefrontal cortex whereby each sensory event is actively retrieved and monitored during rhythmic sequencing (Chen et al, 2009). Therefore we saw marked decrease in synchronization tests performance in cancer patients, who survived after pathological process and severe treatment.
Conclusion
Our results suggest that degree of brain damage after cancer and treatment is associated with ability to synchronize arm movements with auditory cues (PFT>ALL>control). Healthy children are able to anticipate stimuli, but ALL and especially PFT patients mostly reacted after stimulus onset.
Limitations of the study
Different age groups were not analyzed because of the small sample size. It is necessary to look at the developmental changes in rhythmic ability in future research.
Our participants have different history of their disease, so we also have to take into account the period after treatment (remission period)
References
- Bobin-Bègue, A., Droit-Volet, S., & Provasi, J. (2014). Young children’s difficulties in switching from rhythm production to temporal interval production (> 1 s). Frontiers in psychology, 5, 1346.
- Chen, J. L., Penhune, V. B., & Zatorre, R. J. (2008). Moving on time: brain network for auditory-motor synchronization is modulated by rhythm complexity and musical training. Journal of cognitive neuroscience, 20(2), 226-239.
- Droit-Volet, S., Zélanti, P. S., Dellatolas, G., Kieffer, V., El Massioui, N., Brown, B. L., ... & Grill, J. (2013). Time perception in children treated for a cerebellar medulloblastoma. Research in developmental disabilities, 34(1), 480-494.
- Huss, M., Verney, J. P., Fosker, T., Mead, N., & Goswami, U. (2011). Music, rhythm, rise time perception and developmental dyslexia: perception of musical meter predicts reading and phonology. Cortex, 47(6), 674-689.
- Jerde, T. A., Childs, S. K., Handy, S. T., Nagode, J. C., & Pardo, J.V. (2011). Dissociable systems of working memory for rhythm and melody. Neuroimage, 57(4), 1572-1579.
- Kasatkin, V., Shurupova, M., Ryabova, A., Anisimov, V., Kovaleva, A. & RumyantsevA. (2017). Disorders of audio-motor synchronization in patients with cerebellar tumors. Russian Journal of Pediatric Oncology and Hematology. 4, 39-48. DOI: 10.17650/2311-1267-2017-4-3-51-57
- Konoike, N., Kotozaki, Y., Jeong, H., Miyazaki, A., Sakaki, K., Shinada, T., ... & Nakamura, K. (2015). Temporal and motor representation of rhythm in fronto-parietal cortical areas: an fMRI study. PloS one, 10(6), e0130120.
- Knoblich, G., Butterfill, S., & Sebanz, N. (2011). Psychological research on joint action: theory and data. In Psychology of learning and motivation (Vol. 54, pp. 59-101). Academic Press
- Lockwood, K. A., Bell, T. S., & Colegrove Jr, R. W. (1999). Long-term effects of cranial radiation therapy on attention functioning in survivors of childhood leukemia. Journal of Pediatric Psychology.
- Margelisch, K., Studer, M., Ritter, B. C., Steinlin, M., Leibundgut, K., & Heinks, T. (2015). Cognitive dysfunction in children with brain tumors at diagnosis. Pediatric blood & cancer, 62(10), 1805-1812.
- Provasi, J., & Bobin-Bègue, A. (2003). Spontaneous motor tempo and rhythmical synchronisation in 2½-and 4-year-old children. International Journal of Behavioral Development, 27(3), 220-231.
- Provasi, J., Doyère, V., Zélanti, P. S., Kieffer, V., Perdry, H., El Massioui, N., ... & Droit-Volet, S. (2014). Disrupted sensorimotor synchronization, but intact rhythm discrimination, in children treated for a cerebellar medulloblastoma. Research in developmental disabilities, 35(9), 2053-2068.
- Repp, B. H., & Su, Y. H. (2013). Sensorimotor synchronization: a review of recent research (2006–2012). Psychonomic bulletin & review, 20(3), 403-452.
- Saito, S. (2001). The phonological loop and memory for rhythms: An individual differences approach. Memory, 9(4-6), 313-322.
- Schmahmann, J. D. (2004). Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. The Journal of neuropsychiatry and clinical neurosciences, 16(3), 367-378.
- Thomson, J. M., & Goswami, U. (2008). Rhythmic processing in children with developmental dyslexia: auditory and motor rhythms link to reading and spelling. Journal of Physiology-Paris, 102(1-3), 120-129.
Copyright information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
About this article
Publication Date
23 November 2018
Article Doi
eBook ISBN
978-1-80296-048-8
Publisher
Future Academy
Volume
49
Print ISBN (optional)
-
Edition Number
1st Edition
Pages
1-840
Subjects
Educational psychology, child psychology, developmental psychology, cognitive psychology
Cite this article as:
Kovaleva, V., Anisimov, V., & Kasatkin, V. (2018). Audio-Motor Synchronization Tests For Cognitive Functions Assessment In Children With Cns Lesions. In S. Malykh, & E. Nikulchev (Eds.), Psychology and Education - ICPE 2018, vol 49. European Proceedings of Social and Behavioural Sciences (pp. 302-310). Future Academy. https://doi.org/10.15405/epsbs.2018.11.02.34

