Zataria Multiflora Boiss Improves Learning And Memory Impairment Induced By Toxoplasma Gondii Infection
Abstract
Recent epidemiological and experimental studies also showed that latent toxoplasmosis can lead to a number of neurological and behavioral disorders such as learning and memory impairments. Recent studies showed that the essential oil and methanolic extract of the
Keywords: Zataria multiflora" learning" Memory" Toxoplasma gondii
Introduction
Problem Statement
Nowadays it has been proven that tissue cysts are able to influence several biological activities of cells for example synthesis of some neurotransmitters, synapse contrauction, and signaling (Prandovszky, 2011; Gatkowska, 2013). Recent epidemiological and experimental studies also showed that latent toxoplasmosis can lead to a number of neurological and behavioral disorders such as learning and memory impairments (Celik, 2010; Alipour, 2011).
Research Questions
Acetylcholine (ACh) represents a key function in the regulation of cognitive and behavioral activities (Blockland, 1996). Recent investigations exhibited that toxoplasmosis enhances the activity of acetylcholinesterase (AChE) as a membrane-bound enzyme which hydrolyses ACh (Tonin et al, 2013; 2014). Herbal medicines are usually well-known as prosperous source for prevention and treatment of numerous diseases such as behavioral and neurological disorders in the various countries around the world (16).
Purpose of the Study
Here, we evaluated the effect of
Research Methods
5.1. Animals
Thirty two male BALB/c mice (6–8 weeks old) weighing from 20 to 25 g were purchased from the
5.2. Parasite
Here to induce the latent
5.3. Establishment of T. gondii infection
The mice model of latent toxoplasmosis was established based on the method explained by Saraei et al (Saraei, 2014). In brief, 0.5 ml of the brain suspension of infected mice containing the 20-25 tissue cysts was intraperitoneally administrated tested mice.
5.4. MAT test
To confirm the establishment of chronic toxoplasmosis, 60 days post-infection, all the inoculated mice were examined for anti-
5.5. Treatment with ZME
ZME at the dose of 0.1 and 0.2 ml/kg was orally administrated once a day for two weeks starting from post-infection day 90.
5.6. Morris water maze (MWM) test
The MWM task was used to assay spatial learning and memory (Aghaei 2014). The MWM consisted of a black circular swimming pool which was painted with nontoxic materials black circular pool, 160 cm diameter, 80 cm height-filled with water maintained at room temperature to a depth of 40 cm. The pool was geographically divided into four quadrants of equal size and starting points were designated at each quadrant as N, S, E, and W. A square platform (10 cm diameter) was hidden just below (1.5 cm) the surface of the water in the center of the northeast quadrant. The experiments were carried out in a dimly light room with various and fixed extra maze geometric images (e.g., circles, squares or triangles) attached at different points on the walls around the maze. Performances were recorded by a smart video tracing system (Noldus Ethovision® system, version 5, USA) and animals could be traced on the screen of a computer.
5.6.1. Spatial learning
In the spatial acquisition phase, the mice were allowed to find a submerged hidden platform during a 60-second-interval in four training trials (inter-trial interval = 60 s) repeated in three blocks (inter-block interval = 30 min). After finding the platform, the animals were allowed to rest on the platform for 20–30 s. The mice were dried with a towel and returned to their cages. After 20 to 30 s of rest, they were once again put in the chamber for the next trial. When mice did not find the platform within 60 s, the experimenter would put it on the platform. On each trial, mice were randomly released into the water from one of the four quadrants of the maze with their faces toward the wall of the quadrant where they were released. Each mouse had 4 different releasing points. Parameters such as latency and the traveled distance to find the platform were recorded in each trial.
5.6.2. Short term spatial memory
Two hours after the acquisition phase, a probe test was performed to evaluate spatial memory retention. For the probe test, the platform was removed and each mouse was allowed to swim for 60 s. The time and distance spent in the target quadrant (quadrant 4) were analyzed as a measure of spatial memory retention.
5.6.3. Latency to visible platform and swimming speed
Following the probe trial, mice had to complete a visible platform test to determine any possibility of
5.7. Statistical analysis
Obtained results are expressed as the mean ± SEM. Data analysis was carried out by using SPSS statistical package version 17.0 (SPSS Inc., Chicago, IL, USA). One-way ANOVA with Tukey’s post-hoc test was used to assess differences between experimental groups (20). In addition, P<0.05 was considered statistically significant.
Findings
6.1. Latency to visible platform and swimming speed
Table
6.2. Spatial learning
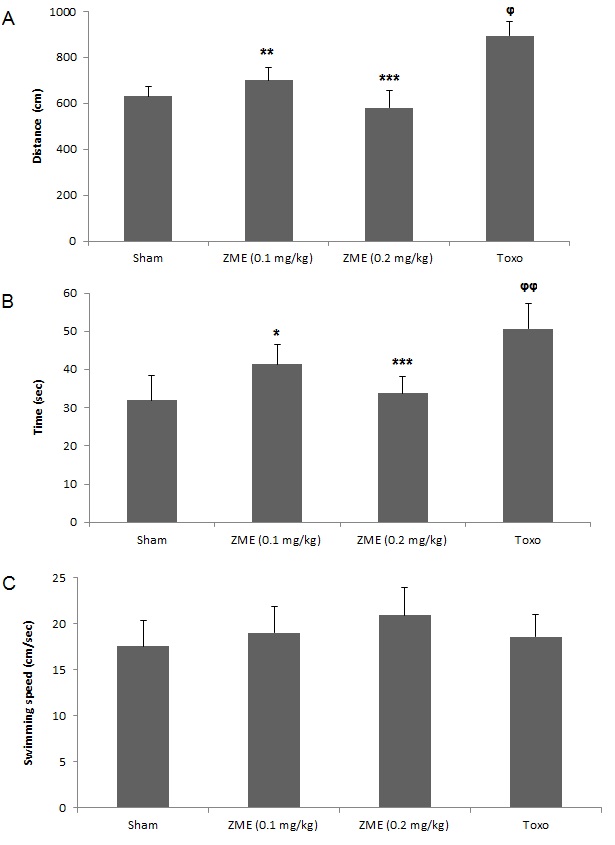
The obtained results showed that the distance traveled to reach the platform was considerably increased in mice with toxoplasmosis (P < 0.05) in comparison with the control group, demonstrating a weaken learning in infected mice. After treatment of infected mice with ZME in doses of 0.1 and 0.2 ml/kg, the distance travelled to reach the platform was decreased compared to the control group (Figure 01A). Figure 01B showed, the escape latency of infected mice significantly (P < 0.01) enlarged compared to mice in the control group; however after treatment infected mice with ZME in doses of 0.1 and 0.2 ml/kg the escape latency significantly reduced (P < 0.05) in comparison to the untreated-infected mice. The statistical analysis also showed that there was no considerable difference in the swimming speed of mice in the the all tested groups (Figure 01C).
6.3. Short term spatial memory
In term of spatial memory, the statistical analysis demonstrated that in the infected mice with
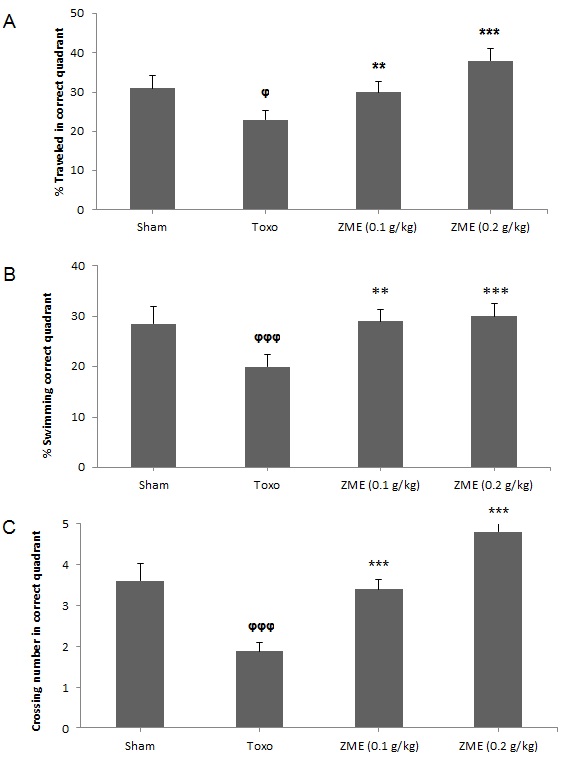
Conclusion
The previous studies demonstrated that
Recently, Tonin et al (2014) have reported that latent toxoplasmosis has ability to influence cholinesterase activity and improve the AChE levels in brain of infected mice (Tonin et al, 2013; Tonin et al 2014). Since last years, reviews have reported that ACh play a chief function in the regulation of learning and memory functions (Blockland, 1996). Nowadays, the successful approaches to treat neurocognitive disorders considered to improve the ACh activity via enhancement of level of Ach level using production promoters and also inhibitors of its metabolizing enzyme. Between the diverse methods studied, the blockage of AChE is the main useful one (Giacobini, 1996; Easton et al., 2013). In the recent years, AChE inhibitors extensively applied to get better the cognitive disorders including learning and memory ones by increase acetylcholine levels at synapses (Giacobini, 1996; Easton, 2013). Recently, Sharififar et al (2012) have reported that the essential oil and methanolic extract of the
Acknowledgments
We would like to thank Dr. Amir Tavakli Kareshk to establish the
References
- Aghaei, I, Shabani, I, Doustar, N, Nazeri, M, Dehpour, A. (2014). Peroxisome proliferator-activated receptor-γ activation attenuates motor and cognition impairments induced by bile duct ligation in a rat model of hepatic cirrhosis. Pharmacol Biochem Behav. 120: 133–139.
- Alipour, A., Shojaee, S., Mohebali, M., Tehranidoost, M., Abdi Masoleh, F., Keshavarz H. (2011). Toxoplasma infection in schizophrenia patients: a comparative study withcontrol group.Iran J Parasitol. 6(2):31-7.
- Azizi, Z., Ebrahimi, S., Saadatfar, E., Kamalinejad, M., Majlessi, N. (2012). Cognitive-enhancing activity of thymol and carvacrol in two rat models of dementia. Behav Pharmacol. 23(3):241-9..
- Blockland, A. (1996). Acetylcholine: a neurotransmitter for learning and memory? Brain Res. 21: 285–300.
- Celik, T., Kamişli, O., Babür, C., Cevik, MO., Oztuna, D., Altinayar, S. (2010). Is there a relationship between Toxoplasma gondii infection and idiopathic Parkinson's disease? Scand J Infect Dis. 2010; 42(8): 604-8.
- Dalimi, A., Abdoli, A. (2012). Latent toxoplasmosis and human. Iranian J parasitool 2012; 2(1): 1-17.
- Daniels BP, Sestito SR, Rouse ST (2015). An expanded task battery in the Morris water maze reveals effects of Toxoplasmagondii infection on learning and memory in rats. Parasitol Int. 2015; 64(1), 5-12.
- Dubey, JP. (2004). Toxoplasmosis-a waterborne zoonosis. Vet Parasitol. 126: 57–72.
- Easton, A., Sankaranarayanan, S., Tanghe, A., Terwel, D., Lin, AX., Hoque, N., Bourin, C., Gu, H., Ahlijanian, M., Bristow, L. (2013). Effects of sub-chronic donepezil on brain Abeta and cognition in a mouse model of Alzheimer’s disease. Psychopharmacology (Berl). 2013; 230 (2): 279– 289.
- Fekadu, A., Shibre, T., Cleare, AJ. (2010). Toxoplasmosis as a cause for behavior disorders–overview of evidence and mechanisms. Folia Parasitol. 2010; 57: 105–113.
- Gatkowska, J., Wieczorek, M., Dziadek, B., Dzitko, K., Dlugonska, H. (2013). Sex-dependent neurotransmitter level changes in brains of Toxoplasma gondii infected mice. Exp Parasitol. 2013; 133: 1–7.
- Giacobini, E. (1996). New trends in cholinergic therapy for Alzheimer’s disease: nicotin agonists or cholinesterases inhibitors? Prog Brain Res. 1996; 109: 311– 323.
- Haroon, F, Handel U, Angenstein, F, Goldschmid,t J, Kreutzmann, P., Lison, H., et al (2012). Toxoplasma gondii actively inhibits neuronal function in chronically infected mice. PLoS One. 2012; 7: e35516.
- Hill, D, Dubey, J. (2002). Toxoplasma gondii: transmission, diagnosis and prevention. Clin Microb Infect. 8(10): 634-640.
- Hosseinzadeh, H., Ramezani, M., Salmani, GA. (2000). Antinociceptive,anti- inflammatory and acute toxicity effects of Zataria multiflora Boiss extractsin mice andrats.Journal of Ethnopharmacology 2000; 73: 379–385.
- Jukic, M, Politeo O, Maksimovic M, Milos M, Milos M (2007). In vitro acetylcholinesterase inhibitory properties of thymol, carvacrol and their derivatives thymoquinone and thymohydroquinone. Phytother Res. 2007; 21(3):259-61.
- Jung, BK, Pyo, KH, Shin, KY, Hwang, YS, Lim, H, Lee, SJ, et al (2012). Toxoplasma gondii infection in the brain inhibits neuronal degeneration and learning and memoryimpairments in a murine model of Alzheimer's disease. PLoS One. 2012; 7(3):e33312.
- Mahmoudvand, H., Saedi Dezaki, E., Soleimani S., Baneshi MR., Kheirandish F., Ezatpour B., Zia-ali N (2015). Seroprevalence and risk factors of Toxoplasma gondii infection among healthy blood donors in southeast of Iran. Parasite Immunol. 2015; 37(7): 362-367.
- Mahmoudvand, H., Ziaali, N., Aghaei, I., Sheibani,, V., Shojaee S, Keshavarz, H., Shabani, M. (2015). The possible association between Toxoplasma gondii infection and risk of anxiety and cognitive disorders in BALB/c mice. Pathogen Glob Health. 2015, 109(8), 369-76.
- Mahmoudvand, H., Ziaali, N., Ghazvini, H., Shojaee, S., Keshavarz, H., Esmaeeilpour, K., Sheibani, V. (2016). Toxoplasma gondii infection promotes neuroinflamation through cytokine networks and induced hyperalgesia in BALB/c mice. Inflamation. 2016, 39(1), 405-412.
- Miman, O., Mutlu EA., Ozcan, O., Atambay, M., Karlidag, R., Unal, S. (2002). Is there any role of Toxoplasma gondii in the etiology of obsessive–compulsive disorder? Psychiatry Res. 2002; 177: 263–265.
- Prandovszky, E., Gaskell E., Martin H., Dubey JP., Webster JP., McConkey G. (2011). The neurotropic parasite Toxoplasma gondii increase dopamine metabolism. PLoS One 2011; 6: e23866.
- Rostami, A., Keshavarz H., Shojaee S., Mohebali M., Meamar AR. (2014). Frequency of Toxoplasma gondii in HIV positive patients from West of Iran by ELISA and PCR. Iran J Parasitol. 2014; 9(4): 474-81.
- SaediDezaki, E., Mahmoudvand, H., Sharififar, F., Fallahi, S., Monzote, L., Ezatkhah, F. (2016). Chemical composition along with anti-leishmanial and cytotoxic activity of Zataria multiflora. Pharm Biol. 2016;54(5):752-8.
- Sajed, H., Sahebkar A., Iranshahi, M. (2013). Zataria multiflora Boiss. (Shirazi thyme)--an ancient condiment with modern pharmaceutical uses.J Ethnopharmacol. 2013;145(3):686-98.
- Saraei, M., Ghaderi, Y., Mosavi, T., Shahnazi, M., Keshavarz, H., Shojaee, S. (2014). Brain cystogenesis capacity of Toxoplasma gondii, avirulent Tehran strain in mice. Asian Pacific J Trop Dis. 2014; 4 (2): 739– 742.
- Sharififar, F., Mirtajadini, M., Azampour, MJ., Zamani, E. (2012). Essential oil and methanolic extract of Zataria multiflora Boiss with anticholinesterase effect. Pak J Biol Sci. 2012 Jan 1;15(1):49-53.
- Tavakoli Kareshk, A., Keyhani, A., Asadi, A., Zia-Ali, N., Mahmoudvand, H., Mohammadi, AR. (2017). Seroprevalence of Toxoplasma gondii infection among childbearing age women in the city Kerman, southeastern Iran. J Parasit Dis. DOI
- TavakoliKareshk, A., Keyhani, A., Mahmoudvand, H., TavakoliOliaei, R., Asadi, A., Andishmand, M., Azzizian, H., Babaei, Z, Zia-Ali, N. (2015). Efficacy of the Buniumpersicum (Boiss) Essential Oil against Acute Toxoplasmosis in Mice Model.Iran J Parasitol 2015;10(4):625-31.
- Tonin, AA., da Silva, AS., Thorstenberg, ML., Castilhos, LG., França, RT., Leal, DB., et al (2013). Influence of Toxoplasma gondii acute infection on cholinesterase activities of Wistar rats. Korean J Parasitol. 2013; 51(4): 421-6.
- Tonin, AA., Da Silva, AS., Thomé, GR, Sangoi, MB, Oliveira, LS, Flores, MM, et al. (2014). Influence of toxoplasmosis on acetylcholinesterase activity, nitric oxide levels and cellularlesion on the brain of mice. Pathol Res Pract. 210(8): 526-32.
- Torrey, E., Bartko, JJ., Yolken, RH. (2012). Toxoplasma gondii and other risk factors for schizophrenia: an update. Schizophr Bull 2012; 38: 642–647.
- Worth, AR., Lymbery, AJ., Thompson, RC. (2013). Adaptive host manipulation by Toxoplasm gondii: fact or fiction? Trends Parasitol. 29: 150-155.
- Yesil Celiktas, O., Girgin, G., Orhan, H., Wichers, HJ., Bedir, E., Vardar Sukan, F. (2007). Screening of free radical scavenging capacity and antioxidant activities of Rosmarinus officinalis extracts with focus on location and harvesting times. European. Food. Res. Technol. 224: 443–451.
- Zhou, YH., Wang, XB., Jiang, SF., Xu, YL., Tao, JP., Zhang, XP., et al (2011). Impairment of learning and memory ability in mice with latent infection of Toxoplasma gondii. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 29(5):333-338.
Copyright information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
About this article
Publication Date
14 September 2017
Article Doi
eBook ISBN
978-1-80296-029-7
Publisher
Future Academy
Volume
30
Print ISBN (optional)
-
Edition Number
1st Edition
Pages
1-376
Subjects
Health, public health, preventive healthcare, preventive care, preventive medicine
Cite this article as:
Mahmoudvand, H., Esmaeelpour, K., Ziaali, N., Khaksarian, M., & Jahanbakhsh, S. (2017). Zataria Multiflora Boiss Improves Learning And Memory Impairment Induced By Toxoplasma Gondii Infection. In Z. Bekirogullari, M. Y. Minas, & R. X. Thambusamy (Eds.), Health and Health Psychology - icH&Hpsy 2017, vol 30. European Proceedings of Social and Behavioural Sciences (pp. 47-57). Future Academy. https://doi.org/10.15405/epsbs.2017.09.5
