Abstract
Realization of an inexpensive and effective detoxification method of lignocellulosic hydrolysates is a prerequisite for reducing the cost of biofuels production. We suggest a cost-effective waste-free method of hydrolysates purification from acetone-butanol inhibitors, which is based on the use of specially adapted activated sludge. We conducted the detoxification of an enzymatic hydrolysate of miscanthus cellulose and an acid hydrolysate of spruce by specially adapted activated sludge of the urban wastewater treatment plants. As a result of purification of the spruce hydrolysate, the concentration of 5-HMF has decreased from 3.06 g/L to 0.0012 g/L, furfural – from 1.3 g/L to 0.0009 g/L. The concentrations of 5-HMF have reduced from 1.03 g/L to 0.0013 g/L and the concentrations of furfural have decreased from 0.71 g/L to 0.0008 g/L. We showed the effectiveness of the method by fermentation of the medium based on treated or untreated hydrolysate by
Keywords: Biological detoxificationlignocellulosic hydrolysatesbiobutanolinhibition of acetone-butanol fermentationactivated sludgeClostridium acetobutylicum ATCC 824
Introduction
Production of motor fuel from alternative renewable sources is an important issue of our time. Recent research interests are oriented mainly to the production of biobutanol produced by fermentation from carbohydrates by acetone-butanol bacteria. A fermentation substrate is a significant factor influencing the cost of butanol production. In an attempt to reduce the cost of butanol, scientists are exploring the possibility to use readily available inexpensive raw materials, namely the hydrolysates of lignocellulosic feedstocks, obtained by hydrolysis of forestry and agricultural residues. The use of lignocellulosic biomass considerably reduces the cost of biofuels and also enables effective utilization of agricultural waste (Qureshi, & Ezeji, 2008).
When hydrolyzed, lignocellulosic materials form simple sugars and several compounds derived from degradation of cellulose, hemicellulose and lignin that inhibit acetone-butanol fermentation, the finished hydrolysate can not be used directly for biochemical processing. The lignocellulosic hydrolysates contain inhibitors which include furan derivatives (5-HMF, furfural), carboxylic acids (acetic, propionic, formic, etc.) and various phenolic compounds (simple phenols, phenol, phenolic, etc.) (Parawira, & Tekere, 2011). Formation of these compounds that inhibit fermentation or the growth of acetone-butanol microorganisms is a major problem associated with production of lignocellulosic biobutanol.
A variety of physicochemical and biological detoxification methods have been proposed to reduce the concentration of the inhibitors in lignocellulose hydrolysates. Physicochemical detoxification methods are carried out by lime treatment (Njoku et al., 2013; Martínez et al., 2000), activated charcoal (Mussatto et al., 2004; Berson et al., 2005), evaporation (Palmqvist, & Hahn-Hagerdal, 2000), ion exchange resins (Cho et al., 2009) or nano-filtration (Sun, & Liu, 2010). The general disadvantage of known methods of physicochemical detoxification is their high cost and/or the formation of large amounts of waste, which increases the burden on the environment.
Among all the detoxification methods, the biological strategies to eliminate inhibitors are most promising. There are removal of furfural and phenolic components by yeast
One of the most efficient detoxification methods of lignocellulosic hydrolysates is treatment with a fungus
The disadvantage of all known biological methods of detoxification is the need for time-consuming procedures for the selection of a biological agent maintaining their functional properties and the development of the necessary biomass growth. Moreover, the selective utilization of toxic components and insufficient activity or complete absence of activity against other toxicants are great disadvantages of these methods of biological detoxification. Besides, many bioagents partially utilize carbohydrates as a carbon source.
An inexpensive and effective detoxification method of lignocellulosic hydrolysates is a prerequisite for reducing the cost of biobutanol production.
The aim of this work is to evaluate the biological method of lignocellulosic hydrolysates detoxification by specially adapted biocenosis of activated sludge for improved biobutanol production.
The aim of this work is implementation of the detoxification method of lignocellulosic hydrolysates by specially adapted activated sludge and evaluation of its effectiveness.
Research methods
In this work, we used a specially adapted microbial consortium of activated sludge which is able to utilize toxic components of lignocellulosic hydrolysates without using carbohydrates as a carbon source. We used activated sludge of the urban wastewater treatment plants for adaptation to the toxic components. The bacterial composition of adapted activated sludge is represented by the groups of bacteria related to
The structure of microbocenosis activated sludge was determined by isolation of pure cultures of microorganisms with definition of its morphological, physiological and biochemical characteristics. We identified the microorganisms according to Bergey`s Manual of Determinative Bacteriology (Holt, & Krieg, 1997).
The adaptation of the activated sludge was carried out in two stages: first, the adaptation to the creosote, and then to inhibitors: phenol – 50 mg/L, sodium acetate – 50 mg/L and formic acid – 35 mg/L. The adaptation was carried out under non-sterile condition at room temperature by aeration of cultural fluid for 2-3 weeks with occasional supplementing of creosote or inhibitors in flasks containing 500 ml of cultural fluid, in which a volumetric dose of the activated sludge was about 30 % (Morozova, & Semyonov, 2016).
At the same time, continuous assessment of the respiratory activity of microbiocenosis was carried out by a respirometry method by the instrument «pH-meter/ionomer/oxygen Multitest IPL-513» of Research and Production Enterprise «Semiko» (Russia).
We also monitored pH of the medium during the biomass growth by pH-meter/ion meter/titrator Multitest IPL-111-1 of Research and Production Enterprise «Semiko» (Russia).
We used an enzymatic hydrolysate of cellulose of miscanthus and an acid hydrolysate of spruce in this study. Their composition is listed in Table
Treatment of the hydrolysates was conducted at room temperature under non-sterile condition for 0.5-2 hours. The hydrolysates were added in a vessel with adapted activated sludge for detoxification. The volumetric dose of the activated sludge was about 25-30 %. At the same time was carried out aeration of the mixture. The process of hydrolysate detoxification was estimated by respirometry method (Vanrolleghem, 2002). The treated hydrolysates were separated from the biomass using sedimentation without filtration. We evaluated the efficiency of detoxification by fermentation of a medium based on treated or untreated hydrolysates and by the chromatographic method.
For fermentation, the medium of the following composition was prepared: treated hydrolysate (source of reducing substances) - 1000 ml, spin with spent grains (source of growth factors) - 60 ml. The medium based on untreated hydrolysate was used as a control. Experiments on the fermentation mediums were conducted in 16 replicates.
An acetone-butanol producing strain of Clostridium acetobutylicum АТСС 824 was used for production of biobutanol from lignocellulose hydrolysates. C. acetobutylicum ATCC 824 was obtained from the Russian National Collection of Industrial Microorganisms. This strain has proteolytic, saccharolytic and amylolytic ability (Croux, Canard & Goma, 1992; Bassam & Hans, 1990; Ounine, Petitdemange, Raval & Gay, 1983).
For inoculation, the spores of this strain in the amount of 3% (vol/vol) were transferred to the fresh medium; after heat shocking at 90 °C for 60 seconds the culture was rapidly cooled. The strain was grown at 36.6С° under anaerobic conditions. Fermentation was estimated in two ways: visually on macroparameters (the separation of the nutrient medium, the rise of the solid fraction, the formation of precipitate, the release rate of gas and foam) (Logotkin, 1958) and by gas chromatography.
The concentration of acetone, butanol, ethanol, furfural and 5-HMF was determined by a gas chromatograph «Chromatec crystal. 5000.2» (Russia).
The data obtained in the experiments were processed using the STATISTICA software, version 6.0. Processing of the results was made using parametric statistical methods by calculating the mean and standard deviation.
Results and discussion
In the first phase of works by long-term adaptation, we got a consortium of microorganisms of the activated sludge able to utilize the creosote, the most toxic components of which are anthracene oil.
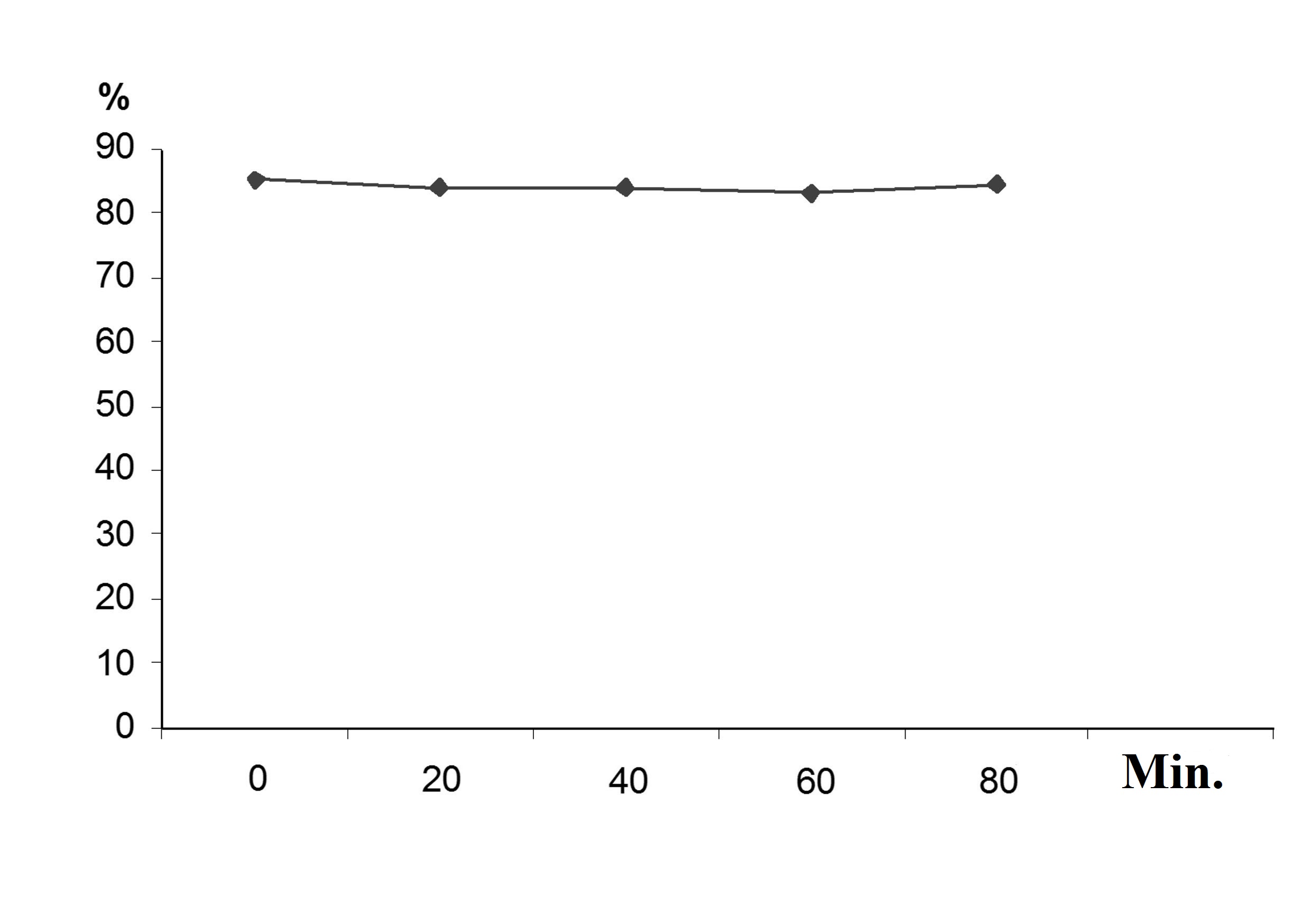
Using a respirometry method, we have verified that the adapted activated sludge does not use carbohydrates as a carbon source. The curve of the dissolved oxygen concentration in the medium proves it (Fig. 1). It shows that the concentration did not change over time in the making of the cultural liquid after adding the solution of reducing substances.
Then we adapted the activated sludge to acetone-butanol inhibitors: phenol, sodium acetate and formic acid. As a result of aeration of activated sludge for seven days with periodic introduction of inhibitors in the culture fluid, the positive dynamics of the absorption rate of dissolved oxygen in the medium was detected. It indicated that the activated sludge has acquired the ability to consume inhibitors as an energy source. However, the rate of utilization of the inhibitors was quite low. It was due to the fact that the pH value was in the strongly alkaline zone. It strongly inhibited a vital activity of microorganisms and biomass growing. Therefore, it was decided to carry out the titration of the medium to achieve permanent neutral values. After stabilization of the active reaction of the culture fluid, the utilization rate of organic substances increased significantly (Fig. 2).
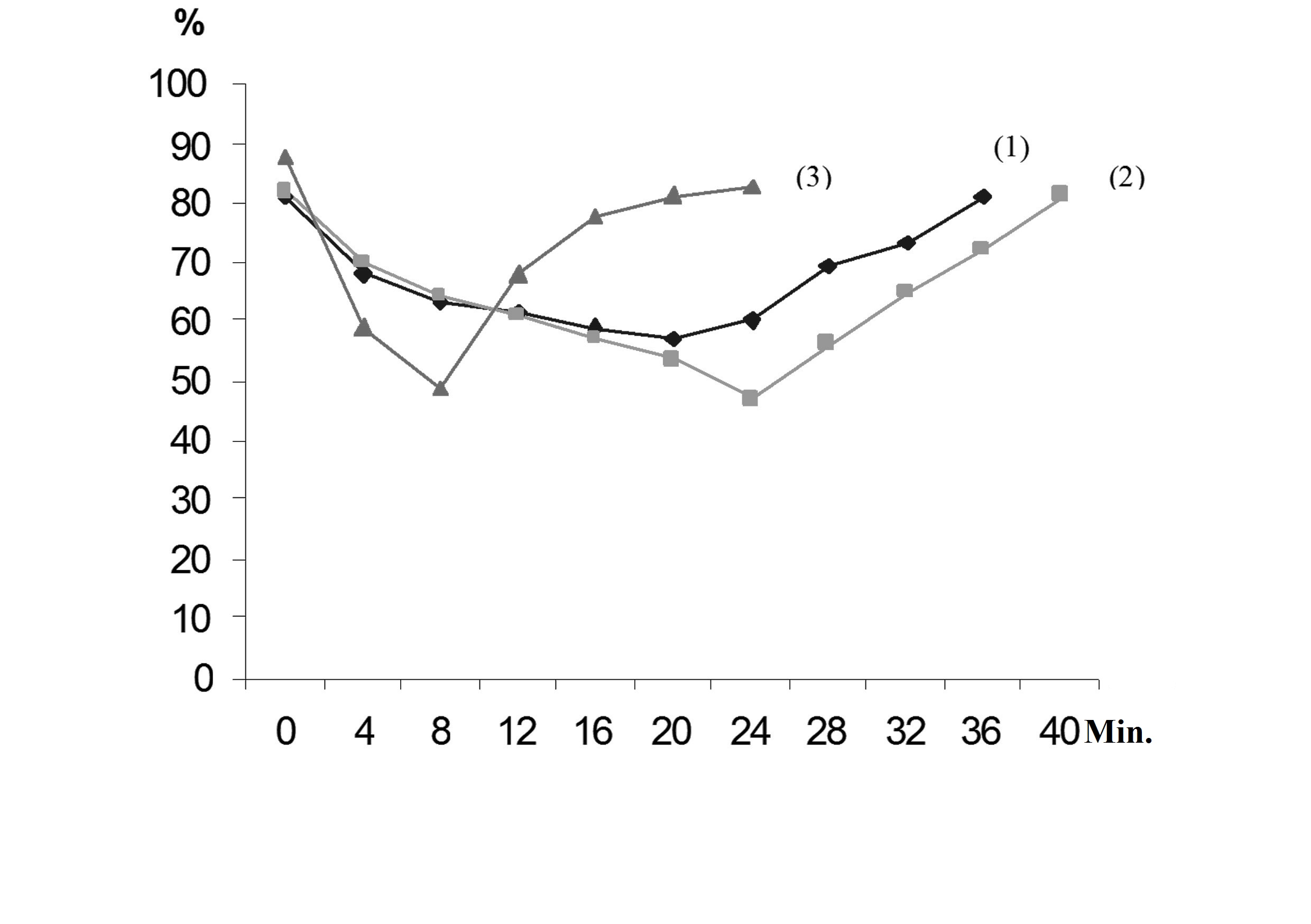
The obtained results pointed to a high activity of the microbiocenosis in the consumption of these substances as an energy substrate. As a result of the adaptation, the activated sludge, which is able to remove inhibitors in 30-60 minutes in concentrations of 0.075%, 0.5-0.7% and 0.0353%, respectively, was obtained.
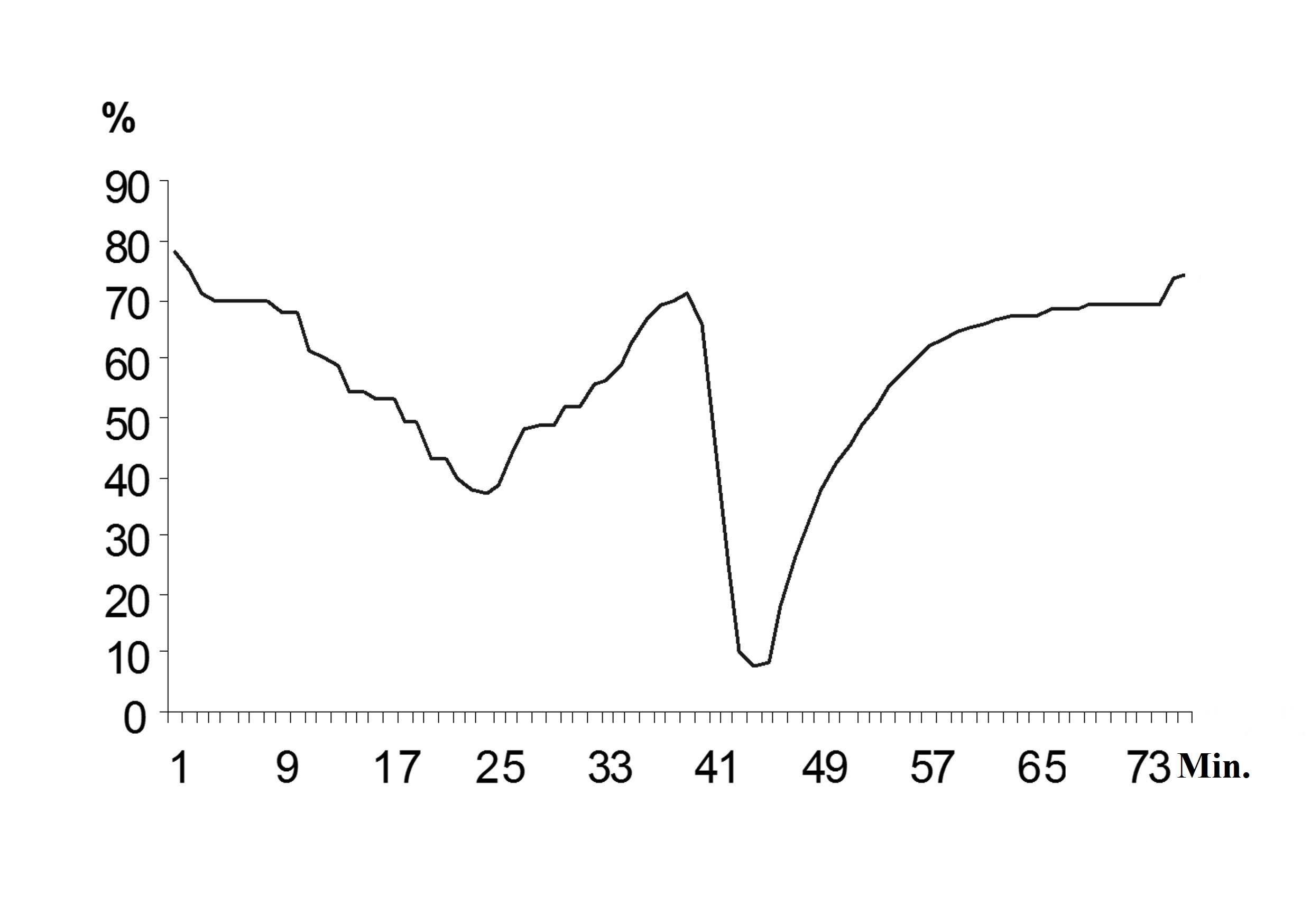
Based on the analysis of chart inhibitors consumption of acetate, formate and phenol, it was concluded that during the first introduction of the mixture of these substances, there was diauxic growth, that is, substrate consumption was carried out sequentially in the order of acetate and phenol, then formate was disposed (Fig. 3).
During the second and subsequent additions, the oxidation of these substances occurred simultaneously (Fig.4).
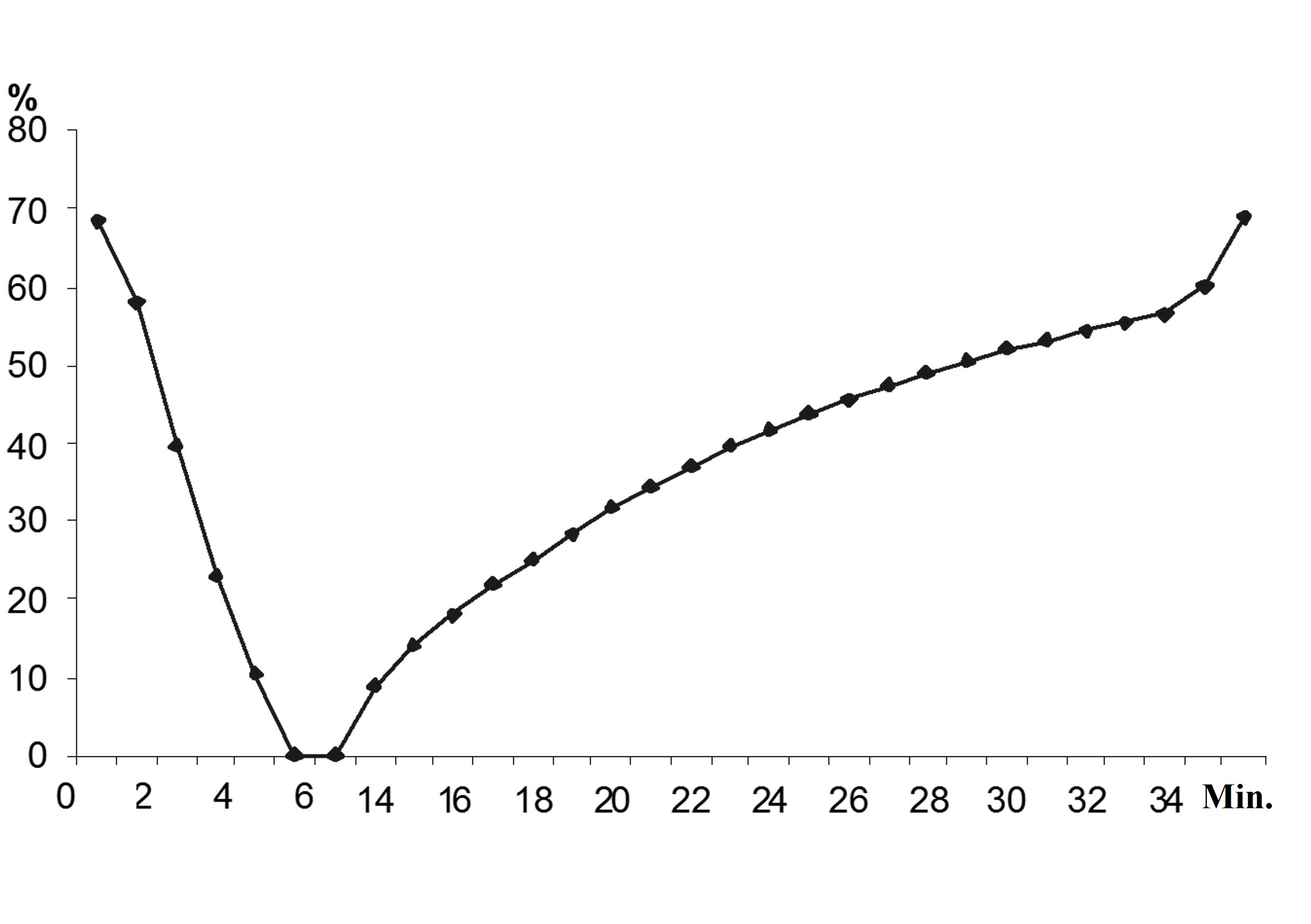
Thus, it was concluded that the adaptation of the activated sludge to new substrates and their concentrations occurred due to the formation of new (inducible) enzyme in microbial cells, and also due to formation of an optimum pool of constitutive and inducible enzymes necessary for the use of such substrates as carbon and energy source.
For a successful detoxification of lignocellulosic hydrolysates, it is necessary to observe a number of conditions, including specific intensity of aeration, which provides the content of oxygen 80-85 % of the saturating concentration prior to the introduction portion of toxic substances. The adaptation of the activated sludge and detoxification of lignocellulosic hydrolysates should be carried out at a temperature of 20-25 °C and in the range of pH 6-8. Also, it is necessary periodically to conduct the activated sludge regeneration, which is carried out by gradually adjusting the pH to the neutral zone by titration with the sulfuric acid for seven days. When culture fluid reaches a neutral pH, it is necessary to perform a two-week repeated adaptation of the activated sludge to inhibitors of acetone butanol fermentation.
In experiments on the detoxification of the lignocellulosic hydrolysates, we used the specially adapted activated sludge separated from the medium by settling. The use of filtration to separate the medium and activated sludge was not required, because the sludge was well flocculating and its settling time does not exceed 20 min. Before treatment of hydrolysates, the testing of the activated sludge for its ability to consume carbohydrates and substance-inhibitors such as sodium acetate, phenol and formic acid was conducted.
Detoxification of the hydrolysate of spruce was carried out at room temperature under unsterile conditions for 60 min., resulting in the concentrations of 5-hydroxymethylfurfural, which was reduced to 0.0012 g/L, and the concentrations of furfural was decreased to 0.0009 g/L. Detoxification of the hydrolysate of cellulose of miscanthus was carried out for 30 min., resulting in the concentrations of 5-hydroxymethylfurfural, which was reduced to 0.0013 g/L, and the concentrations of furfural was decreased to 0.0008 g/L.
Then, we conducted the fermentation of the medium on the basis of these hydrolysates by C. acetobutylicum ATCC 824. The start of fermentation was observed after 8-10 hours, the end of fermentation was noted in 72-80 hours. Control experiments on fermentation of untreated hydrolysates showed a complete absence of fermentation. The fermentation occurred in all 16 replicates and was characterized by intensive processes of gas and foam formation that corresponds to the process of fermentation described in the literature (Logotkin, 1958). Results of experiments on the fermentation of the hydrolysates by the activated sludge indicate high effectiveness of this method of detoxification. The results of the fermentation of the treated hydrolysates by the specially adapted activated sludge of the urban wastewater treatment plants are presented in Table
Conclusions
A novel method to detoxify lignocellulosic hydrolysates to improve the fermentability was investigated in experiments with the specially adapted activated sludge. The obtained results confirmed the effectiveness of the proposed method of detoxification hydrolysates of lignocellulosic materials from fermentation inhibitors. This method is effective, inexpensive and does not require sterile conditions. Lignocellulosic hydrolysates, treated by the proposed method, can be used not only in the production of acetone, but also for the production of ethanol. The main condition for successful detoxification is a long-term adaptation of activated sludge to toxic substances, after which bioagent of detoxification acquires the ability to utilize toxic components of lignocellulosic hydrolysates without using carbohydrates as a carbon source. However, for a successful detoxification of lignocellulosic hydrolysates, it is necessary to observe a number of conditions, including specific intensity of aeration. Also, it is necessary to periodically carry out the regeneration of the activated sludge.
Acknowledgements
The work was partially supported by a program to improve the international competitiveness of Tomsk State University.
References
- Bassam, A. A. & Hans, B. P. (1990). Regulation and Localization of Amylolytic Enzymes in Clostridium Acetobutylicum ATCC 824. Applied and environmental microbiology, 56(8), 2559–2561.
- Berson, E. R., Young, J. S., Kamer, S. N., & Hanley, T. R. (2005). Detoxification of Actual Pretreated Corn Stover Hydrolysate Using Activated Carbon Powder. Applied Biochemistry and Biotechnology, 121-124, 923–934.
- Cho, H. D., Lee, Y. J., Um, Y., Sang, B., & Kim, Y. H. (2009). Detoxification of Model Phenolic Compounds in Lignocellulosic Hydrolysates with Peroxidase for Butanol Production from Clostridium Beijerinckii. Appl Microbiol Biotechnol., 83, 1035–1043.
- Croux, C., Canard, B., & Goma, G. (1992). Autolysis of Clostridium Acetobutylicum ATCC 824. Journal of General Microbiology, 138, 861–869.
- Fonseca, B. G., Moutt, R. O., Ferraz, F. O., Vieira, E. R., Nogueira, A. S., Baratella, B. F., & Silva, S.S. (2010). Biological Detoxification of Different Hemicellulosic Hydrolysates Using Issatchenkia Occidentalis CCTCC M 206097 Yeast. J Ind Microbiol Biotechnol., 26 July 2010, 1–9.
- Holt, J. & Krieg, N. (1997). Bergey`s Manual of Determinative Bacteriology. Russia, Moscow: World, V. 1, 432.
- Logotkin I.S. (1958). Technologies Acetone-Butanol Production. Russia, Moscow: Publishing House Food Industry, 254.
- López, M. J., Nichols, N. N., Dien, B. S., Moreno, J., & Bothast, R. J. (2004). Isolation Of Microorganisms for Biological Detoxification of Lignocellulosic Hydrolysates. Appl Microbiol Biotechnol, 64, 125–131.
- Martínez, A., Rodriguez, M. E., York, S. W., Preston, J. F., & Ingram, L. O. (2000). Effects of Ca(OH)2 Treatments («Overliming») on the Composition and Toxicity of Bagasse Hemicellulose Hydrolysates. Biotech Bioeng., 69, 526–536.
- Morozova, T. S., & Semyonov, S.Y. (2016). Biological Detoxification of Lignocellulosic Hydrolysates for Improved Biobutanol Production. Key Engineering Materials, 683, 525–530.
- Mussatto, S. I., & Roberto, I.C. (2004). Alternatives for Detoxification of Diluted-Acid Lignocellulosic Hydrolyzates for Use in Fermentative Processes: a Review. Bioresource Technology, 93, 1–10.
- Mussatto, S. I., Santos, J. C., & Roberto, I. C. (2004). Effect of Ph and Activated Charcoal Adsorption on Hemicellulosic Hydrolysate Detoxification for Xylitol Production. Journal of Chemical Technology and Biotechnology, 79, 590–596.
- Njoku, S. I., Iversen, J.A., Uellendahl, H. & Ahring, B. K. (2013). Production of Ethanol from Hemicellulose Fraction of Cockfoot Grass Using Pichia Stipitis. Sustainable Chemical Processes, 1:13, 1–7.
- Okuda, N., Soneura, M., Ninomiya, K., Katakura, Y., & Shioya, S. (2008). Biological Detoxification of Waste House Wood Hydrolysate. Journal of Bioscience and Bioengineering, 106 (2), 128–133.
- Ounine, K., Petitdemange, H., Raval, G., & Gay, R. (1983). Acetone-Butanol Production from Pentoses by Clostridium Acetobutylicum. Biotechnology Letters, 5(9), 605–610.
- Palmqvist, E., & Hahn-Hagerdal, B. (2000). Fermentation of Lignocellulosic Hydrolysates. I: Inhibition and Detoxification. Bioresource Technology, 74, 17–24.
- Parawira, W., & Tekere, M. (2011). Biotechnological Strategies to Overcome Inhibitors in Lignocellulose Hydrolysates for Ethanol Production: Review. Critical Reviews in Biotechnology, 31(1), 20–31.
- Qureshi, N., & Ezeji T. C. (2008). Isolation Butanol, «a Superior Biofuel» Production from Agricultural Residues (Renewable Biomass): Recent Progress in Technology. Biofuels, Bioprod. Bioref., 2, 319–330.
- Sun, Z., & Liu, S. (2010). Production of N-Butanol from Concentrated Sugar Maple Hemicellulosic Hydrolysate by Clostridia Acetobutylicum ATCC 824. Biomass and Bioenergy, 30, 1–9.
- Vanrolleghem, P. (2002). Principles of Respirometry in Activated Sludge Wastewater Treatment. Universiteit Gent, Department Of Applied Mathematics, Biometrics And Process Control, 20.
Copyright information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
About this article
Publication Date
20 July 2017
Article Doi
eBook ISBN
978-1-80296-025-9
Publisher
Future Academy
Volume
26
Print ISBN (optional)
Edition Number
1st Edition
Pages
1-1055
Subjects
Business, public relations, innovation, competition
Cite this article as:
Morozova, T. S., Semyonov, S. Y., & Sibataev, A. K. (2017). Biodetoxification of Lignocellulosic Hydrolysates by Specially Adapted Activated Sludge. In K. Anna Yurevna, A. Igor Borisovich, W. Martin de Jong, & M. Nikita Vladimirovich (Eds.), Responsible Research and Innovation, vol 26. European Proceedings of Social and Behavioural Sciences (pp. 690-697). Future Academy. https://doi.org/10.15405/epsbs.2017.07.02.89

