Effect of Peganum harmala L. on lipid metabolism and changes HMGcoA reductase in Hypercholesterolemia-induced male wistar Rat
Abstract
Background: Concentration of cholesterol and other lipids in human diet has been considered as an issue of public health. In the present study the Effects of Peganum harmala (P. harmala) on lipid metabolism were investigated in a 28-days feeding trial. Methods: Total of 64 rats (200±15) were divided into six groups: (G1) control, G2) control plus 100mg/kg, G3) control plus 200mg/kg, G4) control plus 400mg/kg. , G5) 1%cholesterol, G6) 1% cholesterol plus 100mg/kg, G7) 1% cholesterol plus 200mg/kg, G8) 1% cholesterol plus 400mg/kg, In experiment 2, diets G5, G6, G7 and G8a diet of 1% cholesterol were fed stock diet for 4week pre experimental period . The blood and liver tissue samples for serum lipid profile were collected and HMG COA reductase activities determined. Results: P. harmala decreased plasma cholesterol in cholesterol fed rats (100,200,400) mg/kg of the Extract, but decreased plasma triglycerides only in 200,400 mg/kg of the diet group. Peganum harmala supplementation decreased very low density lipoprotein cholesterol and increased
Keywords: Peganum harmala; Lipid metabolism, cholesterol; lipoprotein; HMGcoA reductase gene
Introduction
Hypercholesterolemia is a problem faced by many societies and is a cause of concern for health professionals, since it constitutes one of the major risk factors for the development of cardiovascular diseases, such as atherosclerosis and it`s complications, acute infarction of the myocardium or hypertension (Gomes et al., 1998). In addition, there is a close correlation between these diseases and lipid abnormalities, especially high level of plasma cholesterol, and blood pressure (Mahan et al., 1998). Recently, the effects of dietary components on hypercholesterolemia and atherosclerosis have received much attention. Vegetable proteins, polyunsaturated fatty acids and dietary fiber appeared to lower the blood cholesterol level and events of atherogenic progress (Eini et al., 2014).
Medicinal plants have been used for centuries as remedies for human and animal ailments. They have many pharmacologically active chemical compounds (Chaturvedi et al., 2009), antibacterial (Shahi et al., 2012) and antifungal (Rosinai et al., 2009) agents belong is to the family of and have been shown to possess a diverse range of medicinal properties. Numerous betacarboline alkaloids like harmaline, harmine, harmalol and harmol were present in extract (Herraizi et al., 2010)..harmala extract exhibited great variety of pharmacological and biological activities such as antibacterial and antifungal agents as well as mono amineoxidase (MAO) inhibition and hypothermia. Similarly analgesic, anti-inflammatory (Monsefi et al., 2004), disinfectant (Shahverdii et al. 2005), growth promoting (Qazan et al., 2005), cholesterol lowering and hepato protective effects (Hamdani et al., 2008) have also been reported. Present study was designed to examine the potential benefits of methanolic extract ofresearch article in terms of reducing Protective effects of. On lipid metabolism extract harmine and harmaline against human low density lipoprotein oxidation and also its analgesic effect were studied (Sharaf et al., 1997). The principal compounds of this plant are carboline alkaloids such as harmine, harmaline, harmalol, harman, vasicine and vasicinon (Monsef, 2004). However, it has been reported that contains some flavonoids (Sharaf, 1997) alkaloids have a wide spectrum of pharmacological action let including plate aggregation inhibition (Saeed, 1993), mono amine oxidase inhibition, anxiolyticand behavioral effects; and immune modulator influences (Wang, 1996). There were some reports concerning the cardio vascular actions of harmala alkaloids such as harmine, harmaline and harmalol that reduced systemic arterial blood pressure and total peripheral vascular resistance (Wang, 1997). However,
the antioxidant effect of the major compounds of this plant (harmine and harmaline) and related extracts have not been investigated thoroughly.
Material and methods
The experiment used 64 male Wistar rats each weighing approximately 200±15 mg that was obtained from Academic Center for Education, Culture and Research (ACECR), Qom, Iran. Animals were divided into eight groups in a controlled environment with 12 h light and dark cycles ،٬At 22-25C˚ and humidity 45-50% humidity. The room was lighted from 06:00 to 18:00 hours.
Experiments were conducted. In the first experiment,: G1) control, G2) control plus 100mg/kg, G3) control plus 200mg/kg, G4) control plus 400mg/kg. , G5) 1% cholesterol, G6) 1% cholesterol plus 100mg/kg, G7) 1% cholesterol plus 200mg/kg, G8) 1% cholesterol plus 400mg/kg, In experiment 2, diets G5,G6,G7 and G8a diet of 1% cholesterol were fed stock diet for 4-week pre experimental period .At 12 weeks of age in experiments 1 and 2, diets and water were provided ad libitum. Each dietary treatment group was composed of eight rats in experiments 1 and 2.
2.1 Methanolic extraction of P. harmala P. harmala were collected in the month of May 2013 from desert of Qom city . To obtain methanolic extract 2 kg powder P. harmala of ground was immersed in 10 liters 80 % (v/v) aqueous methanol at room temperature for five days and filtered through Wattmann Filter Paper (No.42). Extraction by Barij Esans co. (Golkaran Ltd.), Kashan City. The extract was then transferred to a glass bottle and stored in refrigerator before use.
2.2. Sample collection At the end of experiments, animals were fasted for 16 hours and weakly anesthetized with phenobarbital. Then, blood samples were taken by cardiac puncture into vacutainer tubes (Becton-Dickinson, Dickinson and Co., Rutherford, NJ). Immediately after blood sampling the liver was excised, washed in chilled saline solution, blotted and cooled in crushed ice. A part of the liver was used for enzyme assay and the rest of the liver was kept at at -165C°.
2.3 Assay kits and Biochemical determinations Plasma glucose concentrations were determined using the Clinical Chemistry system (Pars Azmoon Co, IRAN). Plasma triglycyride was analyzed enzymatically using Tri-Es® (Pars Azmoon Co, IRAN), The separation of plasma lipoproteins and analysis of total plasma cholesterol and each lipoprotein cholesterol were performed using a lipoprotein profiling system (Beckman Instruments, Inc., Fullerton).
2.4. Statistical analysis Data were expressed as mean ± standard deviation. In order to compare the groups, analysis of variance (ANOVA) was used. P < 0.05 values were considered to be statistically significant.
Result and discussion
In this study, we further investigated the protective effect of Peganum harmala on examination
parameters in rat Significant differences (P<0.05) that shown in (Table 1).
Effect of P. harmala L 200,400 mg/kg dosage on average body weight (g) in treatment group
and control group significant differences (P<0.05). (Fig. 1)
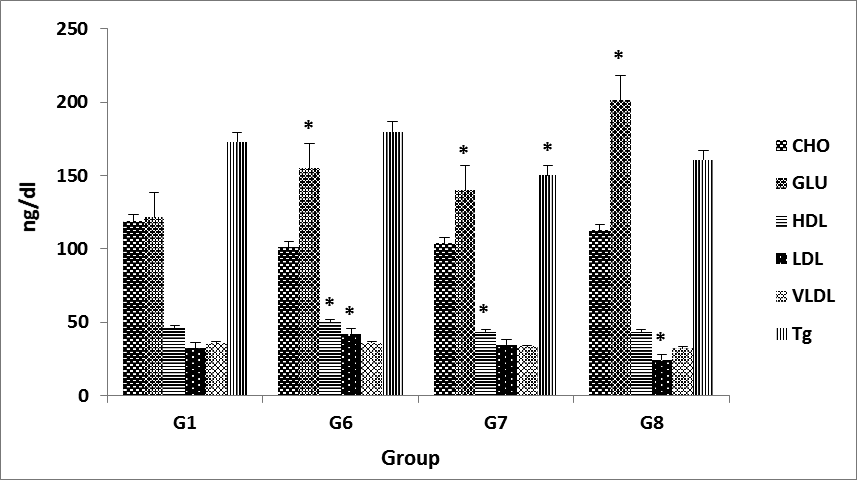
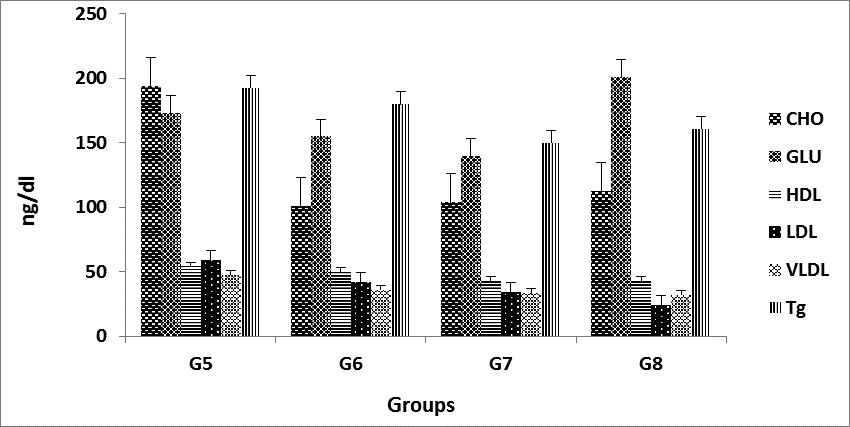
Low density lipoprotein (LDL) and cholesterol and High density lipoprotein (HDL) cholesterol Significant differences (P<0.05) .To our knowledge, no such data has been reported regarding effects of P. harmala on LDL cholesterol, however, effect of other medicinal plants has been reported for LDL decreasing potentials (M. Eini, et al, 2014).
Were recorded between the control group and treated groups as well as among the treated groups at all recorded stages there was gradual increase in the HDL level with increasing level of P. harmala at all recorded stages in all groups. However, other medicinal plant shave been explored for their HDL increasing potential. (fig. 2, & fig. 3) The effect of different levels of P. Harmala methanolic extract on serum vLDL, cholesterol, Glucose Significant differences (P<0.05) in rat is presented in (Table 1, & Fig. 3).
Lowering Effect of P. harmala L 200, 400 mg/kg dosages on Triglyceride in treatment group and control hypercholesteromia and 200 mg/kg dosages compare by control group significant differences (P<0.05) (fig. 2, & fig. 3). HMG COA reductase activities: The P. harmala supplementation further decreased these enzyme activities. P. harmala at the 100 mg/kg dose was similarly effective on lipid metabolism but diffrences was not Significantly, but in, 400 mg/kg doses increased these enzyme activities was Significantly (P>0.05)(Fig. 4, & Fig. 5). The effects of different levels of P. harmala methanolic extract on total cholesterol were presented in (Table 1). Decrease in total cholesterol by 100 mg/kg P. harmala was due to the inhibition of 3-hydroxy-3methyl-glutaryl-CoA (HMGCoA) reductase by different alkaloids harmine, harmaline, and harmol present in P. harmala. These alkaloids have also been reported to have hypoglycemic properties (Singh et al., 2008). So, decrease in Level of Cholesterol in the other groups (200,400 mg/kg P. harmala) that have been associated with increased HMGCoA reductase enzyme levels may be due to the excretion of bile acids. P. harmala has antioxidant property (Jinous and Fereshteh, 2012) which reduces LDL oxidation and thereby reducing total cholesterol content (Abaza, 2001) reported 26-31% reduction of serum cholesterol when supplemented with Trigonella foenumgraecum on mice (Anuja et al., 2012). The mechanism and efficacy of diverse medicinal herbs to reduce serum cholesterol level might be due to the presence of different levels and types of alkaloid.
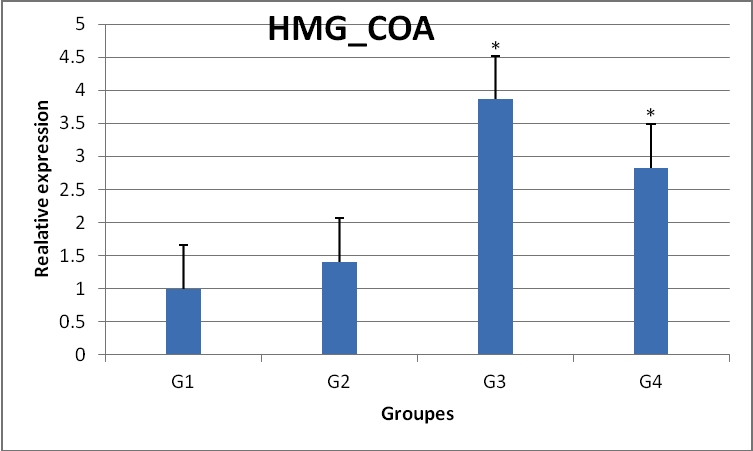
The effects of treatment with harmala extract on the expression of HMG_COA gene is G1) control, G2) control plus 100mg/kg, G3) control plus 200mg/kg, G4) control plus 400mg/kg. The results show that with increasing extract dose, HMG_COA gene expression is increased *) significant difference (P<0.05)
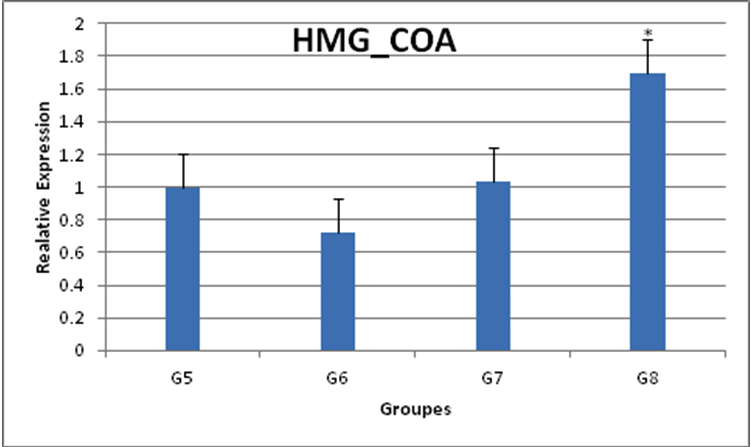
The effects of treatment with harmala extract on the expression of HMG_COA gene is G5) 1%cholesterol, G6) 1% cholesterol plus 100mg/kg, G7) 1% cholesterol plus 200mg/kg, G8) 1% cholesterol plus 400mg/kg
The results show that with increasing extract dose, HMG_COA gene expression is increased P.harmala at the 100 mg/kg dose was similarly effective on lipid metabolism but differences was not Significantly *) significant difference (P<0.05) HMG COA reductase activities: the P. harmala supplementation further decreased these enzyme
activities. P. harmala at the 100 mg/kg dose was similarly effective on lipid metabolism but diffrences was not significantly, but in 200,400 mg/kg doses decreased these enzyme activities was Significantly (P>0.05)
Conclusions
The results of the study that total cholesterol, triglycerides and LDL cholesterol showed gradual
significant decrease with the increasing dose level of P. Harmala up to 250 mgl-1 in drinking water, while HDL cholesterol showed significant increase with increasing level of P. Harmala Symptoms of P. Harmala toxicity experienced by our patient were similar to what had been reported for animals (El-Bahri and Chemli, 1991) and in French case (Salah et al., 1986)However that the cholesterol transport in rats is very different from humans. Rats do not express the CETP enzyme and carry most of their cholesterol in HDL particles (Shapira et al., 1989 and Gupta et al., 1978).
References
Aarons DH, Rossi GV, Orzechowski RF (1977). Cardiovascular actions of three Harmala alkaloids:
harmine, haramaline, and harmalol. J Pharm Sci, 66: 1244-48.
Abdel-Fattah A, Matsumoto K, Murakami Y, El-Hady KA, Mohamed MF, Watanabe H (2006). Facilitato. Inhibitory effects of harmaline on the tryptophan induced 5-hvdroxyt syndrome and body temperature changes in pargyline-pretreated rats. Jpn J Pharmacol, 72: 39-47.
Al-Allaf TA, Khuzaie RF, Rashan LJ, Halaseh WF (1999). Cytotoxic activity of a series of tumor
cell lines with various tumor ligands. Boll. Chem. Pharm, 138: 267-71.
Anuja P, Sunil N, Shashikant P, Vijay T, Manoj T (2012). Anti diabetic effect of polyherbal combinations in STZ induced diabetes involve inhibition of α-amylase and α-glucosidase with amelioration of lipid profile. Phytopharmacology, 2: 46-57.
Berrougui H, Lopez-Lazaro M, Martin-Cordero C, Hmamouchi M, Ettai A (2005). Totoxic activity of methanolic extract and two alkaloidsextracted from seeds of Peganumharmala L. J Nat Remedies, 5: 41-45.
Chaturvedi M, Dwivedi S, Dwivedi A, Barpete PK, Sachan R (2009). Formulation and evaluation
of polyherbal anthelmintic preparation. Ethnobotanical Leaflets, 13: 329-31.
El-Bahri L, Chemli R (1991). Peganumharmala L: a poisonous plant of North Africa. Vet Hum
Toxico, 33: 276-77.
Gomes MR, Tomaso H, Nazarian, GK, Bjarnason H, Dietz Jr, Hunter DW (1998). Upper extremity deep vein thrombosis and chronic pulmonary embolism resulting in pulmonary artery hypertension. Roentgenol, 170: 1532-34.
Hamdan K, Masmoudi H, Ellouz F, Feki AE, Carreau S (2008). Protective effects of
Peganumharmala extracts on thiourea induced diseases in adult male rat. J Env Biol, 29: 73-77.
Herraiz T, González D, Ancín-Azpilicueta C, Arán VJ, Guillén H (2010). Beta-Carboline and Alkaloids in Pegannumharmala and inhibition of human monoamine oxidase (MAO). Food Chem Toxicol, 48: 839-45.
Jinous A, Fereshteh R (2012). Chemistry, pharmacology and medicinal properties of
Peganumharmala L. Afr J Pharm Pharmaco, 6: 1573-80.
Manan A, Chand N, Khan S, Qureshi MS, Rehman AU, Jan B (2012). Effect of periodic supplementation of herbal infusion on the liver function and lipid profile of broiler chickens. Sarhad J Agric, 28: 75-82.
Mohammad Eini A (2014). Effect of Peganum harmala L. on Lipid parameters in hypercholesterolemiainduced male Wistar Rat. Academia Journal of Medicinal Plants. 2(5): 074-078.
Monsef HR, Ghobadi A, Iranshahi M (2004). Antinociceptive effects of Peganumharmala L.alkalid
extract on mouse formalin test. J Pharm Pharmaceut Sci, 7: 65-69.
Qazan WS (2009). The effect of low levels of dietary Peganumharmala L. and Ballotaundulata or
their mixture on chicks. J Anim Vet Adv, 8: 1535-38.
Rosina K, Islam B, Akram M, Shakil S, Ahmad A, Ali SM, Siddiqui M, Khan AU (2009) Antimicrobial activity of five herbal extracts against multi drug resistant (MDR) strains of bacteria and fungus of clinical origin. Molecules, 14: 586-97.
Saeed SA, Farnaz S, Simjee RU, Malik AT (1993). Riterpenes and B-sitosterol from piper betel:
isolation, antiplatelet and anti-inflammatory effect. Biochem Soc Trans, 21(4): 462S.
Shah A, Rasapalli S, Mello C, Singh B R, Cai S (2012). Antibacterial activity of commonly used
traditional Chinese medicines as cold and flu remedies. J Med Plants Res, 6: 234-42.
Shahverdi AR, Monsef-Esfahani HR, Nickavar B, Bitarafan L, Khodaee S, Khoshakhlagh N (2005). Antimicrobial activity and mainchemical composition of two smoke condensates from Peganumharmala seeds. Z Naturforsch C, 60: 707-10.
Shapira Z, Terkel J, Egozi Y, Nyska A, Fiedman J (1989). Abortifacient potential for the epigeal
parts of Peganumharmala. J Ethno-pharmacol, 27: 319-25.
Sharaf M, El-Ansari MA, Matlin SA, Saleh NA (1997). Four flavonoid glycosides from
Peganumharmala. Phytochem, 44: 533-36.
Singh AB, Chaturvedi JP, Narender T, Srivastava AK (2008). Preliminary study on the hypoglycemic effect of Peganumharmalaseeds ethanol extract on normal and streptozocine induced diabetic rats. Indian J Clin Biochem, 23: 391-93.
Tse SYH, Mak IT, Dickens BF (2001). Antioxidative properties of harmane andb-carboline
alkaloids. Biochem Pharmacol, 42: 459-64.
Wang X, Wang H, He A (1996). Study on the antitumor effect of total harmala. J Chin Med Univ,
25: 240-42.
Wang Y, Ohtani K, Kasai R, Yamasaki K (1997). Steroidal saponins from fruits of Tribulus
terrestris. Phytochemistry, 45: 811-17.
Copyright information

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
About this article
Publication Date
09 July 2015
Article Doi
eBook ISBN
978-1-80296-004-4
Publisher
Future Academy
Volume
5
Print ISBN (optional)
-
Edition Number
1st Edition
Pages
1-88
Subjects
Health, psychology, health psychology,health systems, health services, social issues, teenager, children's health, teenager health
Cite this article as:
Kalhor, N., Eini*, A. M., Sharifia, Y., Fazaely, H., & Far, M. A. (2015). Effect of Peganum harmala L. on lipid metabolism and changes HMGcoA reductase in Hypercholesterolemia-induced male wistar Rat. In Z. Bekirogullari, & M. Y. Minas (Eds.), Health and Health Psychology - icH&Hpsy 2015, vol 5. European Proceedings of Social and Behavioural Sciences (pp. 3-11). Future Academy. https://doi.org/10.15405/epsbs.2015.07.2

